These enhancements include the GMP Plant at its main production facility, which is located in Settala (near Milan), Italy. The GMP Plant has been equipped with a state-of-the-art filter dryer for highly potent molecules.
This equipment enables entirely new production runs to be done and means that Indena can meet even more complex and sophisticated client requests — all while keeping their operators safe.
This plant has been approved by AIFA and is now fully operational. Such highly specialised technology is crucial for the production of complex molecules ... for which Indena is recognised as a leading international player.
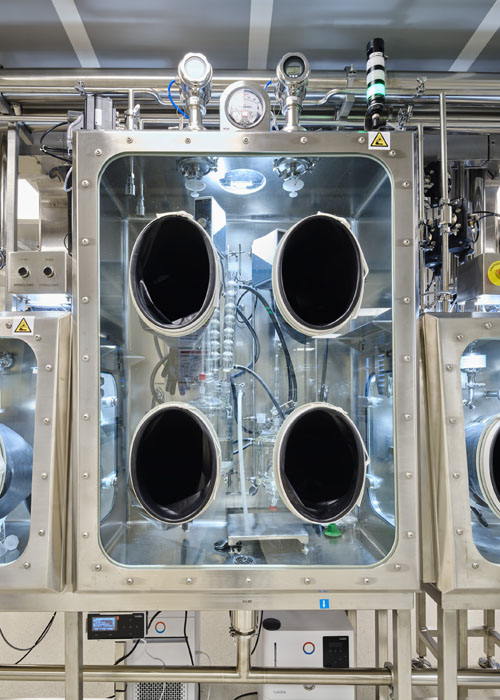
The GMP plant has been expanding since 2024; this growth now means that reaction capacities for chemical syntheses are 10 times larger than before. In addition, HPAPIs (small molecules to OEB5) produced either by synthesis or purification can be made at a greater scale.
The plant can handle a wide range of synthetic reactions at temperatures ranging from –80 to +150 °C.
It includes two 1000 L stainless-steel reactors, 400 and 1000 L glass-lined reactors, a 250 L Hastelloy reactor, a 500 L chromatographic column and a Hastelloy centrifuge, which can also be used for small-scale commercial production.
By the end of 2025, 3000 L enamelled reactors will also be installed, which will enable the large-scale production of APIs and HPAPIs in addition to the smaller-scale projects already under way.
Existing technologies in the GMP Plant — for the chemical synthesis and purification of APIs/HPAPIs — include contained centrifugation (reverse bag centrifuge), contained reactor loading (gloveboxes), isolation and drying with filter dryers featuring surface areas of 0.2, 0.4 and 1.0 m2, and purification with 2000 L chromatography columns operating at pressures up to 9 bar.
For hydrogenation, Indena has recently acquired a 10-bar-compatible Biazzi hydrogenator. Regarding Indena’s facilities, another point of strength is the company’s kilolab LK2 plant at the Settala site.
Started in 2024, LK2 has been further enhanced with equipment that facilitates work with highly potent molecules, thereby ensuring an OEL of 1 ng/m³.
Considering that the previous containment level for highly potent compounds was 20 ng/m³, this is a very significant development.
The site is currently equipped with a freeze-dryer designed to accommodate the production of clinical-scale batches of highly potent compounds.
The uniqueness of Indena’s LK2 facility lies in the fact that both the laboratories and the production plant are located in the same building.
This allows for greater process efficiencies while ensuring the ability to handle highly potent molecules safely.
On the horizon
Looking ahead, two further production lines will be added to research and process highly potent molecules. The first, due to be operational in 2026, will have two reactors (65 and 100 L) — both inside gloveboxes — and a 40 cm diameter Hastelloy C22 filter dryer.
This configuration, with the new equipment integrating with existing systems in the plant, allows for filtration and drying under dynamic conditions.
A second production line will include a freeze-dryer with a surface area of 1.0 m2 and a capacity of 10 kg of ice in 24 hours (also inside a glovebox).
The same glovebox will be equipped with a spray dryer, enabling HPAPI work to be done at exposure levels of less than 1 ng/m³ OEL.

Indena’s most recent expansion programme also includes the construction of a state-of-the-art 400 m2 R&D laboratory equipped with high-performance and high-containment fume hoods (12 benchtop and two walk-in ones), one laminar flow fume hood and two gloveboxes, which can accommodate a large team of scientists.
A new industrial GMP line with reactors up to 10,000 L will allow the company to produce and commercialise many higher-volume APIs and HPAPIs. Moreover, Indena has a fermentation department to run biotransformation or secondary metabolite production processes using live cells.
This can also be used for the in-house production of antibody-drug conjugate (ADC) payload toxins, thereby ensuring an integrated and independent supply chain.
An expert in GMP microbial fermentation and biotransformation, and thanks to the availability of high containment lines for downstream processing, Indena is the ideal partner for precision fermentation-based HPAPI development.
Market growth
Owing to the increasing prevalence of complex diseases and the development of targeted therapies, the market for HPAPIs is growing. It has been estimated that HPAPIs may now account for more than 30% of the drug development pipeline.
Some of the conditions that rely on HPAPIs for treatment include cancer, autoimmune disorders, infectious and rare diseases. Indena remains at the forefront in terms of addressing the latest market challenges.
Among these are molecules that increasingly act in a targeted manner on specific conditions, aligning with the principles of precision medicine.
In the oncology sector, ADCs represent the latest frontier and Indena is actively engaged in research and development activities to produce payload linkers for ADCs on a large scale.
These engineered molecules combine the specificity of monoclonal antibodies with the potent cytotoxicity of chemotherapeutic drugs, thereby offering a more precise and potentially less toxic approach.
People and processes
In terms of providing quality CDMO services, a key factor for Indena is its people. This means the human and professional resources of everyone involved, where lifelong professional experience is fused with the enthusiasm and fresh skills of many young scientists.

Indena understands that highly qualified, motivated and trained technical staff, supported by a corporate culture that’s oriented toward quality and rigorous HSE policies, is essential to ensure that operations run safely, seamlessly and efficiently.
Since the 1990s, Indena has acted as a strategic CDMO partner, going beyond the mere client-supplier relationship and committing to developing new APIs/HPAPIs from early stage clinical batches to commercial-scale volumes.
Indena’s combined technological and research capacity represents a unique and distinctive position in the CDMO sector.
Its leadership and customer service excellence are built on the foundations of continuous investment in new and updated equipment, technologies and talented researchers.
The company’s goal is to enable its customers to run synthetic processes under a wide range of conditions, using either naturally derived molecules (from botanical sources or microbial fermentation), which require semisynthetic steps, or totally synthetic moieties.
Leveraging its analytical, development and manufacturing capabilities, Indena places its services in the “high added value” category by producing complex molecules that require cutting-edge technologies, significant R&D expertise and industrialisation know-how.
Indena is a leading Western European producer of APIs with recognised expertise in highly potent ingredients. The company is equipped to safely manage compounds with OELs as low as 1 ng/m³, regardless of their origin.
Today, HPAPIs represent one of Indena’s core areas of specialisation. The company manufactures 10 commercial HPAPIs and 12 that are currently in clinical development using fully synthetic, semisynthetic and fermentation processes.
Each substance handled by Indena is assigned an OEL and directed to the appropriate production line, ensuring strict compliance with handling and containment requirements.
