During the interview, we covered topics such as the history of Bul Bio, its success in manufacturing biological products in Bulgaria, the importance of “best in class staff” and the benefits of the partnership in terms of furthering the reality of thermostable vaccines. “Our aim is to produce the world’s first fridge-free Td vaccine in partnership with Bul Bio,” said Özgür.
Mr Kofinov explained that Bul Bio is a Bulgarian state-owned company, headed by the Ministry of Health, that dates back as far as 1881. “Our GMP standard facilities export vaccines to more than 100 countries around the world, one of which is a WHO-approved liquid bacterial vaccine for diphtheria and tetanus.” Bul Bio’s portfolio consists of more than 500 products, including human blood plasma products.

Mr Roumen Kofinov
“We are delighted to have had such a strong working relationship with Bul Bio during the last few years. The tetanus and diphtheria vaccine market is expected to reach $7 billion by 2027 and Bul Bio is one of the five WHO prequalified suppliers of the Td vaccine, supplying approximately 9% of global requirements,” added Özgür.
“Worldwide demand for the Td vaccine was estimated to be around 270 million doses in 2019 and continues to grow, mainly driven by WHO’s recommendation in 2015 of moving from tetanus alone to the Td combination vaccine. WHO projects the demand to grow to 410 million doses by 2030,” added Mr Tuncer.
“The production of combined vaccines against diphtheria and tetanus in Bulgaria began in 1956–1958,” explained Mr Kofinov, “and the tetanus-containing vaccines we produce at our GMP site meet all of the regulatory standards issued by the WHO and the European Pharmacopoeia. It’s important to reiterate that much of our success as a business is because of our greatest asset — our people. Let’s be honest, maintaining standards as a state-of-the-art drug manufacturing plant is impossible without adherence to strict operational procedures. Bul Bio employs over 400 specialised staff.”
Doing business in Bulgaria
The British Embassy and DIT in Bulgaria were instrumental in engineering the partnership between Stablepharma and Bul Bio, which has proven to be a great success. “There are real benefits for UK companies to do business in Bulgaria,” said Dr Rob Dixon British Ambassador to Bulgaria.
“A highly skilled, multilingual workforce, low corporation tax (10%) and financial incentives for some investment projects have attracted prominent brands in the pharma and healthcare sector.”
“Stablepharma approached us with a non-standard and revolutionary idea to thermally stabilise the classic tetanus and diphtheria vaccine,” explained Mr Kofinov: “During the last 6 months, we have been conducting a series of in vivo challenge trials with Stablepharma’s thermostable product, StablevaX, according to strict WHO protocols."
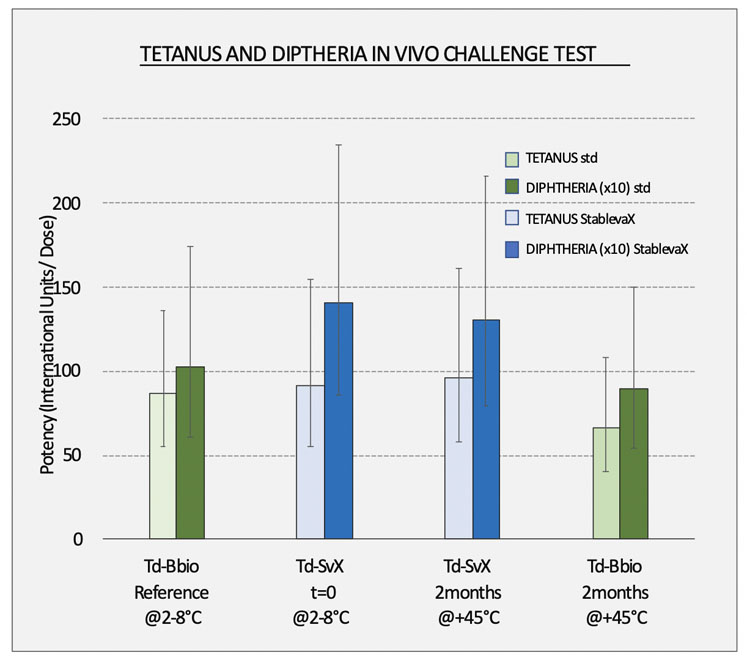
Figure 1: Bul Bio has conducted a series of potency tests with suitable animal models according to EU Ph 2.7.6. and SOP-QC005; it has been shown that a StablevaX reformulation has equivalent potency to the original WHO-approved vaccine and that StablevaX, when kept at 45 °C for 2 months, has equivalent potency in as the refrigerated Tetadif vaccine
"The results of the studies — done using Td StablevaX prototypes that contain the reformulated Tetadif (Bul Bio) vaccine and controls — continue to prove that a thermally stable StablevaX version of the standard Td vaccine,shows no deviations in potency compared with the original prototypes,” said Mr Kofinov (Figure 1).
“Stablepharma’s R&D team is delighted with the execution of the animal challenge potency trials being done in partnership with Bul Bio. They are proving to be a fantastic partner and the resulting data is very encouraging, essentially validating the high-temperature stability of Td-StablevaX for prolonged periods of time with no loss of potency. We’re very excited to be one step closer to fridge-free vaccines,” said Arcadio Garcia de Castro, Chief Scientific Officer, Stablepharma.
“The StablevaX invention is the culmination of decades of work by Stablepharma founder, Dr Bruce Roser, who focused on the preservation of perishable vaccines that require constant and precise refrigeration from the factory to the patient. The WHO estimates that about 50% of all vaccines are wasted; if we can contribute to solving that problem, with the help of our partners at Bul Bio, we can alleviate significant suffering and save many lives. This would be a tremendous outcome for all of us,” commented Mr Tuncer.
Bul Bio is continuously looking to expand its portfolio of products, which requires significant investment in equipment, innovation and expansion of their production facilities. The Bulgarian Government recently provided funding of €11.4 million to support Bul Bio with its ongoing development.
The power of partnership
Mr Kofinov reiterated the enormous benefits of partnerships within the global biotech sector: “This really helps to address the challenges of providing a better quality of life for all societies. Advances in medical innovation should be available to everyone, everywhere. This is a complex issue and one that requires long-term commitment from governments and the private sector.”

Özgür Tuncer (centre) with Dr Arcadio Garcia de Castro, Chief Scientific Officer, and Juana de la Torre Arrietas, Senior R&D Manager, Spain
Özgür Tuncer added: “Stablepharma is a biotech platform built on strategic collaborations with vaccine manufacturers, academia and other non-profit government entities. We were fortunate enough to add several other significant partnerships recently, including Bul Bio, the University of Strathclyde and the University of Southampton, in addition to NIBSC and Innovate UK. Partnerships have got to be the way forward for better healthcare.”
“We have established a strategic R&D partnership, alongside a statement of intention for the future development and commercialisation of the StablevaX Td product, which is proving to be an excellent and exciting programme for both parties,” said Dr Bruce Roser, Chairman and cofounder.
“As British Ambassador to Bulgaria, I am very pleased that we are able to support this great collaboration between our two countries,” said Dr Rob Dixon: “The UK is a world leader in science with extensive expertise in disease outbreaks and vaccine development, whereas Bulgaria is beyond doubt a country with a strong tradition of medicine and vaccine production.”
In summary, Özgür Tuncer commented: “Stablepharma is very pleased to have Bul Bio as a strategic partner as we prepare to launch the world’s first fridge-free Td vaccine.”
