BES has completed a viral vector suite facility for Cobra Biologics; a biologics specialist involved in COVID-19 vaccine scale-up and manufacturing.
Located within Keele University campus, Cobra has invested in upgrading part of its existing development and production facility with 3 viral vector suites to enable the company to expand its clinical and commercial viral vector services as a Contract Development and Manufacturing Organisation (CDMO). The time-critical project is a pivotal element of the company's commercial strategy and BES has delivered the project four weeks ahead of schedule, despite the social distancing and supply chain challenges of the COVID-19 pandemic.
Compliant to ACDP Hazard Group and US BioSafety Level 2, along with FDA, EMA and MHRA standards, the new facilities will allow Cobra to work with customers to develop gene therapy treatments for a range of life-limiting and life-threatening conditions.
Working closely with the Cobra team, BES was responsible for providing design development of the Cobra's concept around their user requirements, leveraging the company's multi-disciplinary team across all mechanical and electrical engineering, architectural design, construction and project management requirements. BES provided a consistent team and an accountable critical path throughout the design and construction phases of the project with clarity on costs and timescales from the outset.
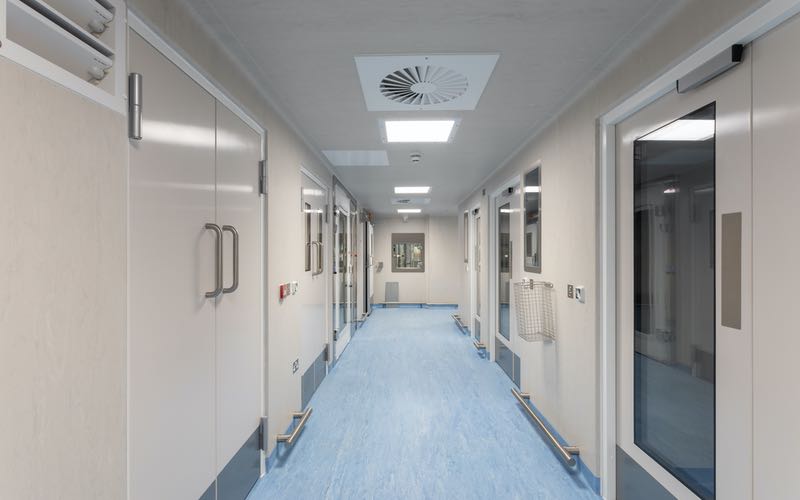
Cobra's clean corridor
The refurbishment project involved design and delivery of three grade C processing suites, each specified to contain a class 2 microbiological safety cabinet (MBSC), which will allow biological manipulations to be carried out in a grade A environment. Scientists will enter the new facilities via a primary change area to gain access to a grade C change area. Processing suites will be accessed via a clean corridor and exited into a 'dirty' corridor that leads back to the primary change area.
The scope of works also included a new extension to the existing building to upgrade the reception lobby. BES implemented a temporary entrance and reception to enable the company to operate as usual while this was constructed.
Amongst the challenging elements of the project was the need to co-ordinate works in order to minimise the risk of business interruption. BES worked closely with Cobra and the university to co-ordinate the relocation of existing cryogenically frozen preparations stored within the area of the building re-purposed as the new viral vector suites. A new secure compound was established for the liquid nitrogen and the BES team meticulously planned and implemented the relocation of these assets to ensure any risk to existing product stocks. Similarly, the new High Voltage (HV) connection needed to accommodate the upgraded facilities was carried out over a weekend to overcome any downtime.
BES has now carried out a successful OQ (Operational Qualification), including particles, pressures, Dispersed Oil Particulate (DOP) tests and clean up rates. Cobra will now carry out final validation and PQ (Performance Qualification) to enable full production to begin for new and existing customers.
Peter Coleman, CEO of Cobra, said: "From the outset, the BES team understood our commercial and operational requirements and worked with us to design and programme the project around those needs."
"The company's experience in the pharmaceutical industry makes them ideally placed to deliver complex projects like this for the gene cell therapy sector," Coleman added. "Their multi-disciplinary team not only worked collaboratively with us, but seamlessly with each other, for a joined-up and efficient approach. This was particularly important during a period when we are involved in development and manufacturing work of international importance for COVID-19 response. It's extraordinary that, despite the challenges of the lockdown, BES has completed the project to such a high standard ahead of schedule."
