Sandy Munro, Nikki Willis and Geraldine Venthoye of Vectura, discuss the factors that should be considered. Selecting the most appropriate delivery platform for an inhaled drug product depends on a wide range of factors, including technical, commercial, manufacturing and the demographics of the target patient population.
These factors must be evaluated afresh for each new development programme and the target delivery technology selected in a “device agnostic” manner.
The physical properties of the active pharmaceutical ingredient (API) will be a major consideration: whether it is a small molecule API or a biologic, whether it’s readily solubilised and the likely dose range. There are also questions of time and expense, including the cost and availability of the API, especially in the early stages of development, as well as the cost of any device and likely volumes.
When working with a new chemical entity (NCE) that has an uncertain future, it is beneficial to work in the fastest and most cost-effective manner in the early stages. At this phase, success in proof-of-concept studies is most important, so dosing flexibility, speed and careful cost management are the key driving forces and may not necessarily be done using the same delivery platform as the commercially launched product.
Case study: optimising lung deposition
Smart nebulisation can be a very effective way of developing a product to the early clinical stage, consuming minimal material to get the best probability of success.
For example, a small molecule development programme for a niche disease required a highly water-soluble molecule to consistently target very high and deep lung deposition from patient to patient with a simple and straightforward formulation development process.
The molecule was in the higher dose range, making it potentially less amenable to being formulated as a dry powder inhaler (DPI), although a capsule format might also have been suitable.
A handheld mesh nebuliser was chosen as it allowed all of the pre-Phase I clinical study pharmaceutical development work to be completed with only a small quantity of material (100 g).
This was sufficient to conduct all of the formulation and analytical method development work, as well as phase-appropriate validation, stability testing and product performance characterisation studies; the latter studies were for a high dose (10 mg). There was only the need to dissolve as much drug as required for the immediate testing that needed to be done, meaning that expensive or scarce API was not wasted during early development.
In silico lung deposition modelling work showed that smart jet nebulisers were superior to conventional jet nebulisers in terms of lung deposition, and also significantly better than a high-performing DPI (Figure 1). Twice as much drug was deposited in the central airways compared with the conventional system and almost four times as much in the smaller airways.
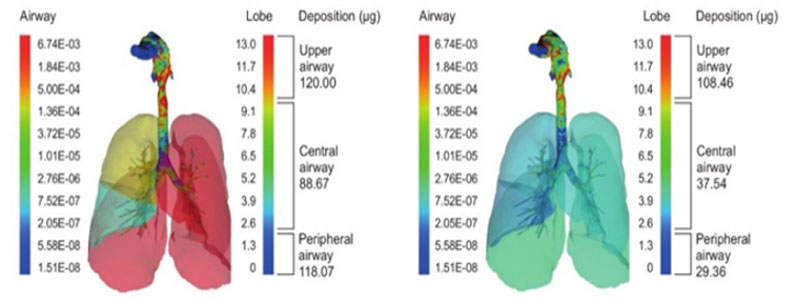
Figure 1: In silico lung deposition modelling for a smart jet nebuliser (left) versus a conventional jet nebuliser (right)
This difference could maximise the probability of success in the early stages of development: the greater the amount of drug that could be dosed to the lungs and the more reproducibly that was achieved, the better the chances of a positive outcome.
A smart nebuliser approach also offered the potential for more consistent delivery because the patient was guided to take every breath by the device. In the mesh nebuliser system described above, the dose sat on top of the mesh and, typically, more than 90% of the dose was delivered to the patient.
A further advantage of smart nebulisation was the ability to deliver a variety of different clinical doses via only two solution strengths by dispensing different volumes into the nebuliser.
Scale-up challenges
Whatever delivery platform is chosen must also be fit for purpose from the perspective of both design and manufacturing. It is beneficial to minimise material requirements and costs, and look to mitigate risks associated with the scale-up of the manufacturing process. One of the dilemmas, particularly in the respiratory field wherein reliable scale-up can be difficult to achieve, is deciding what constitutes an appropriate scale at each stage of development.
In early development phases, when materials are often in limited supply, there is an inevitable trade-off between the scale of manufacture and the number of batches that can be produced to build scientific understanding. Manufacture at the intended commercial scale involves large batch sizes, which can be time-consuming and prohibitively expensive to be practical until the later stages of development.
However, having assurance of the ability to scale-up the manufacturing processes is key to successfully developing products. Using a validated blend scale-up model in the development of a generic DPI treatment for asthma and chronic obstructive pulmonary disease (COPD) shows that it is possible to achieve comparable drug product performance from batches made at 500 g (laboratory blend scale) and batches made at 30 kg (commercial blend scale). See Figures 2 and 3.
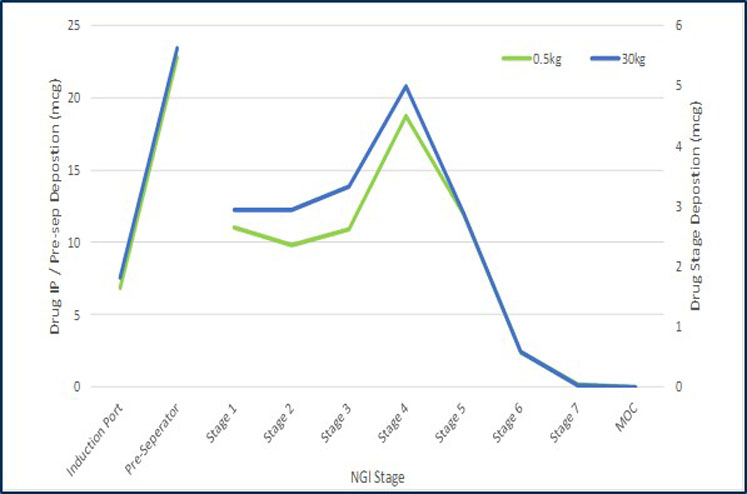
Figure 2: Comparison of the particle size distribution of the emitted dose at two different scales measured by inertial impaction
This model enables a significant amount of the development work to be conducted in the laboratory at development scale, minimising material costs and enabling faster execution of experiments, whilst giving confidence in the ability to move to the larger scale at a later date. It also means that capital investment at the commercial supply manufacturing site could be made at a later development stage based on a risk-based approach for the project.
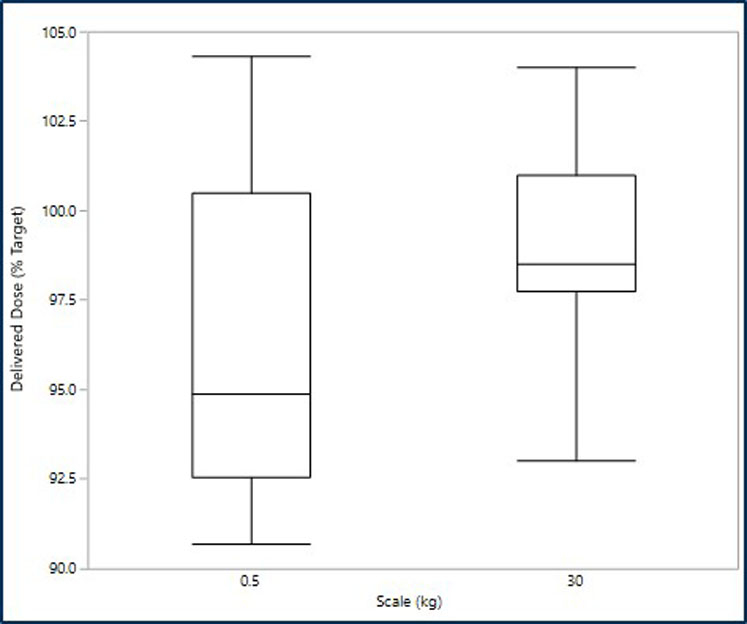
Figure 3: Comparison of the emitted dose at the two scales
Delivery platform
The choice of delivery platform also needs to consider the nature of the disease and the needs of the target patient population. Consideration should be given to whether the therapy will be delivered at home or in hospital, whether delivery needs to be to the deep lung or the central airways and the number of doses per day.
The choice should also take into account the age range of patients, any dexterity or cognitive issues that could influence device usability and compliance with dosing, lifestyle considerations and patient expectations based on other available devices.
Platform technology objectives may vary through the development process; a single platform choice may not necessarily be appropriate for the duration of a programme. Making informed choices at a given stage of development can save time and money and can maximise the probability of a project’s success.
Whilst keeping with the same platform throughout development programmes may be preferred, for some it may be more appropriate to change the delivery platform from the one used in the earlier stages of development, even up to proof-of-concept, to the one that might ultimately be commercialised.
Ideally, a development project would yield a well characterised, commercially ready device, process and formulation at the pivotal clinical stages of development. However, in reality, it is not until a commercial process has been bedded in, many more batches manufactured and hundreds of thousands of devices delivered into the hands of patients that the product becomes more fully understood.
Lifecycle considerations
Rolling out a product across multiple geographical markets increases patient access and drives up volume, which may lead to the need to scale-up production; this usually provides opportunities to reduce costs and helps to maintain a positive margin position, even in adverse or competitive pricing environments.
It is vital for commercial success to maximise volumes and capacity, yields, process capability and efficiencies through synergies and economies of scale, and to maximise supply chain flexibility while minimising risk, obsolescence, cycle times, downtime, costs and working capital. It is also essential to eliminate redundancy, waste and excess inventory with time.
A global supply chain and procurement function is therefore key within any development company that commercialises a product to manage the forecasting, logistics, cost management, contractual and business continuity elements for the product with the company, ensuring that it effectively manages inventory — including safety stock and distribution to its clients and ultimately to patients.
This discipline and experience bring great value when managing situations that threaten to disrupt the supply of critical medicines.
Significant experience in all stages of the development lifecycle and applying the best technology solutions at each stage can help organisations to successfully navigate an inhaled development programme from feasibility through all stages of development and on to commercialisation.
Choosing the correct device or technology platform should be based not only on the needs of the patient and the disease treatment, but also on opportunities to accelerate the proof-of-concept or early clinical stages by using fast-to-clinic approaches. When these considerations are combined with seamless scalability by designing in manufacturability and choosing an appropriate production strategy, projects can be accelerated through later-stage development.
In the commercial phase, continued assessment and evaluation will enable the product to evolve, grow and maximise its potential through focused continuous improvement. Feeding the accumulated know-how and experience of the commercial phase back to development teams will aid industrialisation, minimise cost-of-goods and build robustness into future products.
