Caroline Ailhas, Pharmaceutical Development Director, and Helen Caddy-Leach, Head of Business Development Drug Product at CARBOGEN AMCIS, explore the intricate process of selecting between liquid and lyophilised formulations and the impact of these decisions on stability, cost and product development timelines.
Initial steps in formulation: liquid formulation and solubility testing
Whether the final product is liquid or lyophilised, the development process begins with a liquid formulation; even lyophilised products start life in liquid form before being freeze-dried.
One of the first steps in this process is to conduct a solubility study to understand how the product behaves when dissolved in a liquid.
Solubility studies often start with a standard aqueous solution, which is generally easier for patient administration than solvent-based formulations.
However, depending on the client’s requirements, it may be necessary to increase the concentration to achieve therapeutic efficacy.

The laboratory team must also consider the product’s sensitivity to pH and specific salts and stability with time, which can significantly influence the overall development pathway.
Once the initial solubility testing is complete, the next step is to test the product’s solubility in different aqueous media and determine whether it remains stable under standard conditions.
Key considerations include keeping the pH between 5 and 7 to ensure minimal patient discomfort during injection. At this stage, controlling the salt concentration is also essential to achieve the desired solubility and stability.
Stability as a crucial determinant for formulation selection
Stability testing is critical when deciding whether a product can remain liquid or must be lyophilised. A product that remains stable for 24–72 hours may not present significant issues; but, if stability cannot be maintained beyond a week, lyophilisation is likely necessary.
If the solution stays stable for up to a week and meets the client’s expectations in terms of concentration, further enhancements are undertaken. This involves refining buffer strengths, adjusting pH levels and potentially introducing surfactants to improve solubility and stability.
Regulatory and quality by design considerations
Regulatory considerations also play a critical role in formulation development. Every choice, whether buffer selection, pH adjustments or salt concentration, must be justified to regulators.

It would be necessary, for example, to explain why “50 units of a compound were chosen instead of 25” based on the product's behaviour during testing.
At CARBOGEN AMCIS, the quality by design (QbD) approach is a standard part of the development process. QbD principles are incorporated early on to proactively address regulatory concerns.
By doing so, the development team ensures smoother approval pathways and better aligns with the client’s long-term goals. Prestability testing is another essential phase.
In these early stages, a product is typically exposed to non-ICH and accelerated conditions to gauge its durability with time. This preliminary testing provides valuable insights into whether the product can remain as a liquid or if lyophilisation is necessary.
Prestability testing and the role of solvent-based formulations
Aqueous-based formulations are often preferred for their ease of use and stability. However, some APIs (active pharmaceutical ingredients) are insoluble in water, necessitating solvent-based formulations.
Although effective for specific cases, these solvent-based formulations present their own challenges — particularly for injectables. The FDA’s approved solvents list is relatively limited. Moreover, solvent-based products cannot typically be lyophilised as freeze-drying is problematic for these formulations.
Therefore, a thorough evaluation of both solubility and stability is crucial during prestability testing. Clients often face tough decisions at this stage: is it worth continuing with a solvent-based liquid formulation or should they pursue lyophilisation?
Understanding these trade-offs is essential to make informed decisions that might impact the drug’s cost and development timeline.
Patient considerations: the role of the formulation in clinical settings
When deciding on a formulation, the method of administration to patients must also be considered. In most clinical settings, drugs are diluted in saline bags for delivery. This introduces another layer of complexity: will the product precipitate when diluted in saline?

What happens when a highly insoluble product is placed into aqueous media? For CARBOGEN AMCIS, patient-friendliness is often less of a concern than stability and regulatory compliance.
Approximately 90% of the drugs handled are oncology treatments, which are typically administered by healthcare professionals in hospitals. Stability and regulatory compliance are the primary foci … as well as the customer’s willingness to invest time in its development.
Lyophilisation: a strategic choice for long-term stability
Many clients, particularly those in early clinical stages, are focused on speed and managing costs. Their goal is to demonstrate efficacy quickly, often opting for frozen liquid formulations in Phase I and delaying the development of a lyophilised formulation until Phase II or III.
However, this approach comes with risks. Although lyophilisation is more expensive in terms of development and manufacturing time, investing earlier has advantages.
Lyophilisation offers several benefits compared with liquid formulations, particularly regarding long-term stability and improved shelf-life. Temperature-sensitive products can benefit significantly from lyophilisation, allowing storage at 2–8 °C or room temperature rather than –20 °C.
This temperature flexibility reduces the logistical complexities of packaging and transporting the product and eliminates the need for cold chain management — a significant cost consideration and concern in large-scale production.
Optimising the lyophilisation cycle
Once a liquid formulation is deemed to be unsuitable for long-term storage, lyophilisation becomes necessary. The first step in this transition involves modifying the formula slightly, such as adding a bulking agent like sugar.
These agents stabilise the product during the freeze-drying process, creating a “nice and elegant cake” and a stable, lyophilised form of the drug. Additional studies are conducted to build on the developing knowledge base.
Initial lyophilisation cycles tend to be conservative, often taking up to a week. However, this timeline is unsustainable in a GMP environment, wherein time equals money.
The goal is to optimise the cycle, reducing it to 2–3 days maximum whenever possible. However, the API often determines the length of the lyophilisation process.
Some APIs are more challenging to freeze-dry than others and the product’s physical properties often determine the cycle’s duration.
Nevertheless, efforts to optimise the process are essential as reducing the lyophilisation cycle significantly impacts production efficiency and cost. Precise physical data is critical to achieve this.
By addressing challenges early in the development process and fostering open dialogue, CDMOs can build trust and help their clients to avoid costly surprises later in the drug’s lifecycle.
A robust, well-optimised lyophilisation process reduces the likelihood of formulation issues arising in Phase II or III, saving time and money.
Overcoming common client concerns about lyophilisation
For some clients, lyophilisation is seen as both costly and complex. However, reconstituting a lyophilised product is usually straightforward and, once this step is complete, the product behaves precisely like a liquid formulation.
This hesitation often stems from a lack of familiarity with the process, particularly for companies that are new to lyophilisation. Cost is another factor; many clients prefer liquid formulations because they perceive lyophilisation to be too expensive.
However, experienced clients, especially those working with highly potent compounds, recognise that lyophilisation can provide significant benefits in terms of stability and product longevity … and ask for lyophilisation from the start.
The trend towards lyophilised formulations is primarily driven by increasingly complex products, such as antibody-drug conjugates (ADCs). ADCs are highly sensitive and notoriously difficult to stabilise in liquid form.
Although future advances may lead to more stable liquid ADC formulations, lyophilisation remains the preferred option for these drugs (Figure 1).
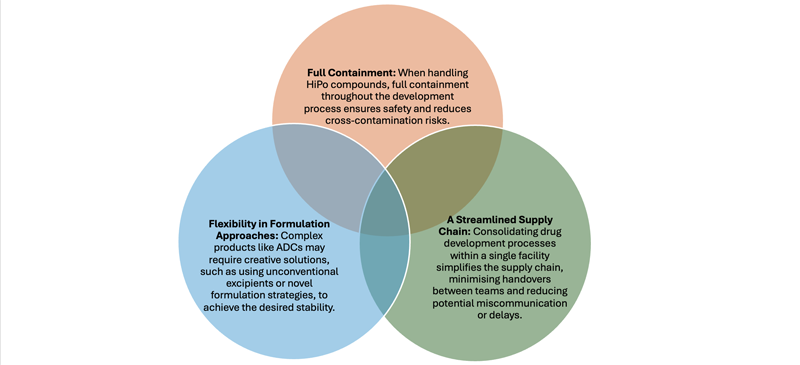
Figure 1: Key requirements to develop and manufacture lyophilised products for HiPo compounds
Case study: addressing a formulation challenge
An excellent example comes from a project with a start-up company. The client initially developed the formulation elsewhere but, when they attempted to manufacture their clinical batch, they encountered significant issues.
They urgently needed to rework the formulation, so they contacted CARBOGEN AMCIS for help. Leveraging the available in-house capabilities, the multidisciplinary team started to rebuild the formulation from scratch.
By looking beyond standard excipients and exploring less conventional options, they resolved the formulation issue and enabled the client to move forward with their clinical batch.
This shift in strategy and out-of-the-box approach is a hallmark of the company’s problem-solving ethos and highlights the importance of creativity when overcoming formulation challenges.
Expertise and collaboration
Delivering effective solutions requires collaboration between experts from different fields. A team with diverse professional backgrounds — from bioanalysis to pharmaceutical manufacturing — can provide fresh insights and lead to creative problem solving.
This diversity fosters innovative solutions that more homogenous teams might overlook. Regular communication between development and manufacturing teams is crucial.
Seamless transitions, shared knowledge and ongoing data tracking ensure that issues are addressed efficiently and delays are avoided. Additionally, revisiting earlier development stages allows for adjustments without compromising timelines.
Simplified in-house approach
Maintaining an integrated and streamlined process is essential to delivering a high-quality product.
Housing more stages of development under one roof reduces risks of lost knowledge or repeated tasks.
Retaining expertise within the same teams ensures that challenges are handled effectively and product consistency is maintained from development to release.
Conclusion
Successfully navigating the challenges of highly potent compounds and lyophilised products requires key capabilities: full containment, streamlined supply chains, flexibility in formulation and a collaborative team.
These factors ensure that the product is safe, effective and meets both client and regulatory expectations.

