A part of Thermo Fisher’s Pharma Services Group, Patheon is a North Carolina-based contract development and manufacturing organisation (CDMO) that has invested $26 million to update a sterile pharmaceutical development services (PDS) suite and to build a fully integrated sterile PDS suite at its site in Greenville, North Carolina.
Further expansion
At its large, multipurpose pharmaceutical manufacturing and packaging campus in Greenville, Patheon is also investing in facilities that will expand its packaging and serialisation capabilities. It will also add a continuous manufacturing suite and new stability storage to support the company’s North American facilities. GMP-compliant Patheon has designed the updated PDS facilities to be compliant with regulatory authority requirements and provide 7000 ft2 of GMP sterile manufacturing space.
It will manufacture sterile liquid and lyophilised drug products in the newly built PDS facility, which is fitted out with freeze dryers and a fully integrated filling line with a restricted access barrier system (RABS) for sterile drug products.
The RABS offers flexibility in turnaround time, while maintaining a high level of containment and separation from the environment (Figure 1).
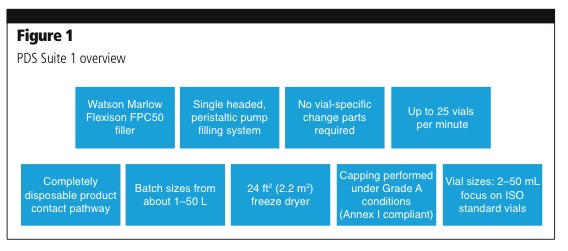
The new facility was designed by leveraging network resources from Patheon’s corporate engineering experts and other global facilities, particularly the company’s manufacturing site in Ferentino, Italy, which also has a PDS suite for investigational medical products. Building on insight and experience gained from the Italian site, Patheon saw and understood that client needs frequently involve a number of different sterile formats, which led the company to build the Greenville PDS facility with technologies of varying capabilities that best accommodate client and industry demands. The Greenville site also offers two manufacturing suites in the new PDS area that operate with a 100% disposable product pathway.
