Widely acknowledged to be a predictable vehicle for the control, distribution and transportation of therapeutic agents around the body, engineered formulations make a variety of common and emerging controlled release dosage forms possible. These include fixed-dose combinations, combined, modified release encapsulated forms and multi-unit particulate system tablet designs.
In this article, Holger Shen takes a look at how this dynamic technology is applied in manufacturing, the sophisticated dosage forms that this process helps to create and why it supports the delivery of on-target APIs and patient outcomes.
Development of patient-centric drug strategies accelerating
Delivering higher therapeutic performance and better health outcomes to patients are central drivers of contemporary drug development. According to an industry whitepaper on patient centricity, the shift is primarily because of pharma’s growing focus on value-based care.1
Now prioritising quality over quantity, the healthcare industry and its payers have eschewed fee-for-service models and are adopting approaches that are centred on improved patient health outcomes and better patient experiences.
Regulators have also been playing a greater role in integrating the patient’s voice into drug R&D.For instance, under the Prescription Drug User Fee Act (PDUFA V), the US Food and Drug Administration (FDA) conducted 24 disease-specific Patient-Focused Drug Development (PFDD) meetings to systematically gather patients’ perspectives on their condition and available therapies to treat their condition.
The report, covering all 24 meetings, was released at the end of 2019.2 Similarly, the European Medicines Agency (EMA) maintains its Patient Engagement Framework for guidance and thought leadership, with support from the European Patients’ Academy (EUPATI).3
For the most part, regulators and developers are looking for more effective ways to incorporate patient reported outcomes to understand the value that new or redeveloped therapies can bring to a specific modality or treatment area.
Ultimately, the overarching reason regulators reoriented and prioritised the emphasis of pharmaceutical development on the patient was to improve the overall economics of pharmaceutical-based healthcare.
Extrusion-spheronisation purpose-built for complex OSD development
Extrusion-spheronisation (ES) is proving to be an extremely versatile granulation technique and a virtually purpose-built enabling technology for many of today’s most popular but complex OSD development paths, including new molecular entities (NMEs) and lifecycle management (LCM) extensions (Figure 1).
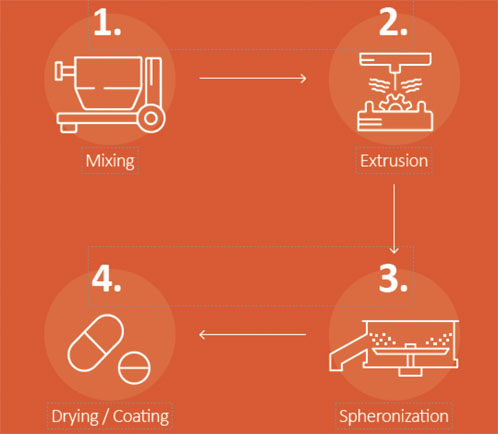
Figure 1: Spheronisation’s four simple steps: mixing, extrusion, spheronisation and drying/coating.
Because of its broad utility in OSD engineering, ES granulation methods also support emerging 505(b)(2) development pathways by tailoring existing compounds to deliver better therapeutic performance, dosing convenience or to treat new indications.
The accelerated 505(b)(2) pathway, for example, could support the development of a paediatric version of a drug formerly indicated to only treat adults. ES granulation could also be leveraged to design fixed-dose combinations (FDCs) and create a best-in-class product and better patient outcomes.
Extrusion-spheronisation’s extra strength dose-control potential
Patient-centricity is manifesting itself across the life sciences industry in many fundamental ways, but where the movement is being realised most profoundly is in the formulation and manufacture of OSD medications. Tailoring dosage forms to better suit a given patient population’s special needs and treat disease more effectively — in principle — are all patient-centric.
Increasingly, pharma is turning to expert outsourcing partners to deliver the advanced processing and manufacturing capabilities they need to create, commercialise and finish these innovative drug products.
Modified release formulations, for example, represent an effective way to deliver drug substances that have a narrow therapeutic index or need to be in an OSD form to ensure dose compliance. Orally administered drugs are by far the preferred dosage form; most patients, regardless of demographics, respond well to taking oral medications, especially ones that are easy to swallow, require fewer doses to be effective and that reduce or eliminate unpleasant side-effects.
Development of ES during the past decade has been accelerated by pharmaceutical science’s more comprehensive understanding of the raw materials, active ingredients, processes and controls behind the methodology. Understanding how to manage and manipulate those inputs to increase therapeutic performance or modify API release — without compromising the integrity of the compound or molecule — has also grown significantly.
The dynamics of extrusion and subsequent spheronisation can be managed closely to control solubility and bioavailability characteristics, including manipulating the blending of size and density to attain different release patterns with time. Multiparticle, multisphere finished-dosage forms offer drug developers economic ways to explore and exploit complex patient-centric OSD drug strategies (Figure 2).
Multiparticle systems are proving to demonstrate higher levels of desired reproducible PK and PD behaviours (including insoluble potent APIs), resulting in improved therapeutic effect in patients. For drug designers looking to develop patient-centric OSDs, these finished dose forms offer new levels of flexibility and utility.
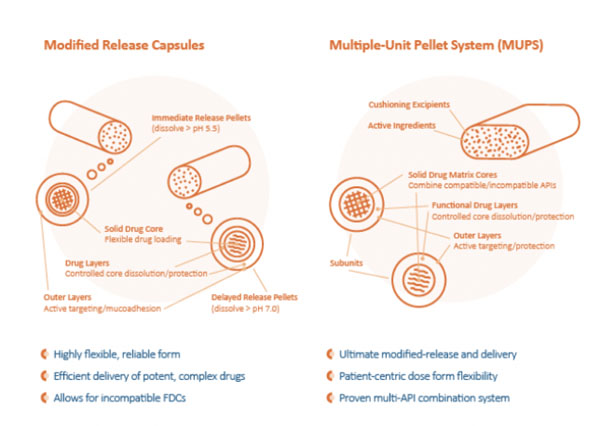
Figure 2: The benefits of multiparticle/multisphere finished-dosage forms
The modified release dose control that ES allows can also be used to help diminish or eliminate side-effects, manage irritation or discomfort in the gastrointestinal (GI) tract and/or target release in the lower intestine to avoid acidic environments.
Why ES is such practical best practice
Offering processing accuracy and quality control, ES offers inherent efficiency, reliability and minimum variability. Because of the efficiencies related to drug loading and similar characteristics in formulation, the process generates economies on all fronts without diminishing quality.
For example, single APIs often require dispersion throughout a solid matrix to control release. These matrices can limit the rate at which active ingredients are dispersed into the body. The process also offers a reliable, flexible alternative to other spheronisation or pellet agglomeration manufacturing techniques such as coating or layering.
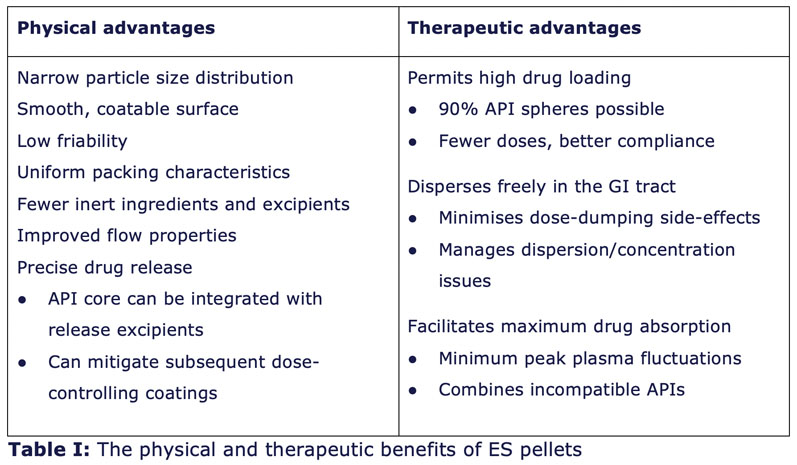
Further, drug pelleting via ES is acknowledged as a much faster process than drug layering. Extruded-spheronised pellets offer distinct physical and therapeutic advantages to drug designers (Table I).
Seek experienced manufacturing partners for best results
Pharma’s development pipeline is being filled with more sophisticated, harder to make and administer compounds. To achieve therapeutic goals and patient and payer value, these formulations require complex delivery strategies to deliver actives efficiently and effectively for the best possible patient outcomes.
As the development and commercialisation of complex OSD products continue to soar, so too will demand for expert experienced allies with the capabilities, capacity and specialised solutions to manufacture and commercialise these products.
For its part, ES has demonstrated tremendous success in improving the quality, safety and therapeutic efficacy of today’s commercial drug products. The process can also be leveraged to improve dose compliance and other key performance indicators relative to the therapeutic value of the drug.
Delivering therapeutic compounds and molecules via spheronised forms will continue to offer pharma the utility and functionality they need to meet their most visionary drug product manufacturing goals.
Focus on extrusion-spheronisation in manufacturing. In the 1970s Reynolds demonstrated that the ability of ES to produce spheres with higher drug loading was one of the primary advantages of the process. At present, it is possible to achieve upwards of 95% drug loading with this technique, depending on the API. The process is easily scalable and repeatable, as well as capable of providing a consistent and uniform product. Since its introduction, ES has experienced significant development as the industry has come to understand how well the technique can be engineered to support controlled API delivery in OSD forms.
Where drug technology and drug strategy meet
A first-in-class route: For one prospective drug owner, the API in the formulation of their first-in-class oncology treatment had both solubility and bioavailability issues.⁴ Single fixed-release forms would require a three-times-a-day dosing regimen that simply was not feasible from a dose-compliance and overall therapeutic value standpoint.
These challenges were overcome by leveraging the unique flexibility of spheronisation to modify and control the release of API during an extended period within a multiple-unit pellet system (MUPS) form. The result: an easy-to-swallow coated tablet that, in a single dose, enabled the controlled release of highly potent active ingredients during a 24-hour period.
A best-in-class FDC strategy: Facing the patent expiry of a lucrative product, a drug developer was seeking to explore a 505(b)(2) lifecycle management strategy that involved integrating two proven but incompatible APIs into a single dose, controlled-release capsule.
The challenge for this company’s development team was to create a best-in-class therapy that bettered patient outcomes and therapeutic performance compared with multiple doses of the two medications.⁴ Spheronisation’s ability to segregate and control previously incompatible APIs and combine other functional ingredients in cohesive pellets within a single OSD form offered a solution.
References
- www.zs.com/-/media/pdfs/pharmas-road-map-to-patient-centricity.pdf?la=en.
- www.fda.gov/industry/prescription-drug-user-fee-amendments/voice-patient-series-reports-fdas-patient-focused-drug-development-initiative.
- www.eupati.eu/.
- https://social.eyeforpharma.com/column/which-more-important-commercial-success-first-market-or-best-market.
