The DSC thermogram of pure RTV showed a sharp endothermic peak at 122.12 °C, which was attributed to melting of the crystalline drug (data not shown), whereas the DOE extrudates showed no endothermic or exothermic events, indicating that the extrudates were rendered amorphous. Example DSC thermograms of batches 2 and 6 are shown in Figure 3a and 3b, respectively. It can be seen that even at the low processing temperature and short residence time, a successful amorphous system was obtained (Figure 3a).
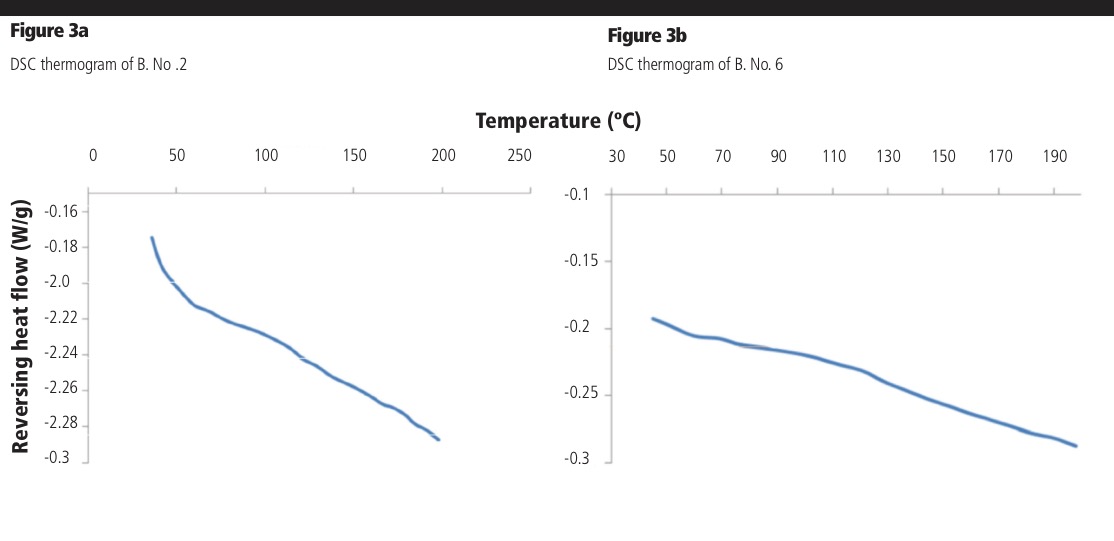
The Tg of pure amorphous RTV is 45–49 °C (onset to endpoint, Patent EP1418174B1); this event was not observed in any DOE batch, indicating the absence of drug-rich domains. The predicted glass transition temperature of the 1:2 RTV:AFF formulation is approximately 84 °C as calculated using the Gordon-Taylor equation; however, the DOE batches each display a Tg at 73.9 °C (Figures 3a and 3b). This negative deviation from the predicted value is attributed to weak RTV:AFF interactions, resulting in a positive entropy of mixing.1 Despite this negative deviation from the predicted value, the single observed Tg indicates good miscibility between the two components.
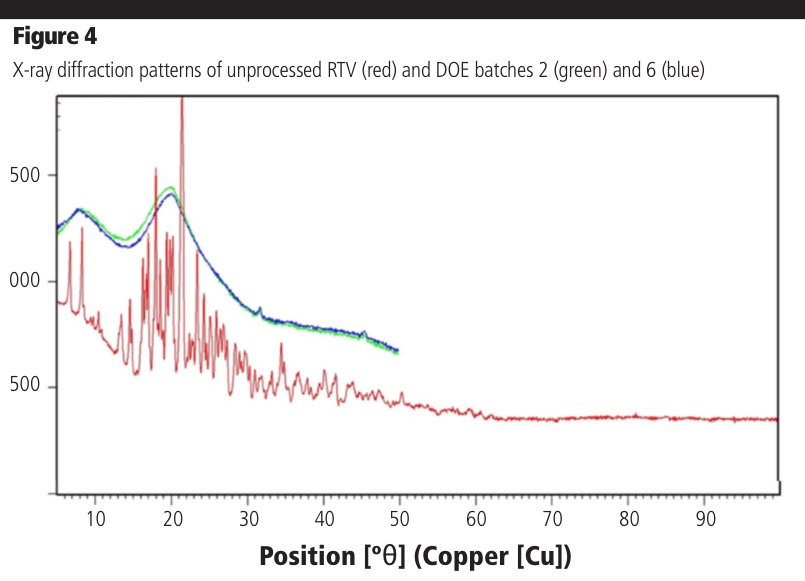
Powder X-ray diffraction: The XRD diffractogram of pure RTV showed characteristic crystalline peaks between 2θ of 5–45°. However, the extruded samples did not show crystalline peaks in this range and displayed only an amorphous halo. This was true for all conditions of the DOE, including the low temperature conditions, confirming that processing parameters did not impact the amorphous nature of the extrudates in good agreement with the DSC data.
The XRD of batches 2 and 6 are shown in Figure 4 as examples. The amorphicity of the low temperature samples also demonstrates a strong capability of the polymer to solubilise the drug into the polymeric matrix. The minor peaks in the extrudates at approximately 32 °C and 45 °C are derived from residual sodium chloride present in HPMC following manufacture.
Drug content
The RTV content was determined by HPLC for all extruded batches of the DOE to understand the impact of processing conditions on drug stability. The drug recovery values of the DOE batches are shown in Table II; primary impurities were not quantified. The results showed that RTV degradation occurred as the temperature increased from 130 to 170 °C. This result was expected, owing to the known thermal instability of RTV.
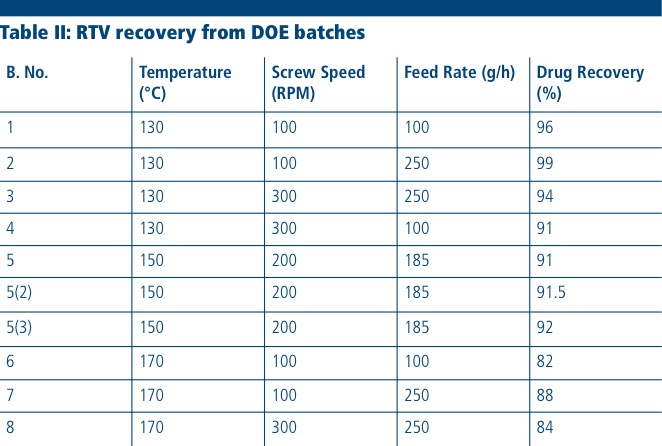
Fitting the data confirmed that temperature, screw speed and feed rate all have a statistically significant impact on drug degradation (p <0.05) with a rank order of temperature > feed rate > screw speed. Thus, it was observed that at the most aggressive DOE conditions utilising the maximum screw speed (300 RPM), longest residence time (100 g/h) and highest temperature (170 °C), RTV showed the greatest degradation (>20%) while the extrudates obtained at the least aggressive DOE conditions of 130 °C, a feed rate of 250 g/h and a speed of 100 RPM resulted in the least degradation (~1%).
For a drug with process sensitivity challenges such as RTV, the ability of AFFINISOL HPMC HME to be extruded across a broad temperature range allows the selection of acceptable extrusion conditions to reduce or eliminate drug degradation.
Dissolution studies
Ritonavir release profiles of extruded DOE batches are shown in Figure 5. Because of the impact of processing conditions on drug degradation, differences in dissolution profiles were observed. For example, the extrudate obtained at 130 °C, a feed rate of 250 g/h at 100 RPM showed ~70% release in 60 min in 0.1 N HCl, whereas the extrudate prepared at 170 °C, a 100 g/h feed rate and 100 RPM screw speed showed approximately 55% drug release in 60 min.
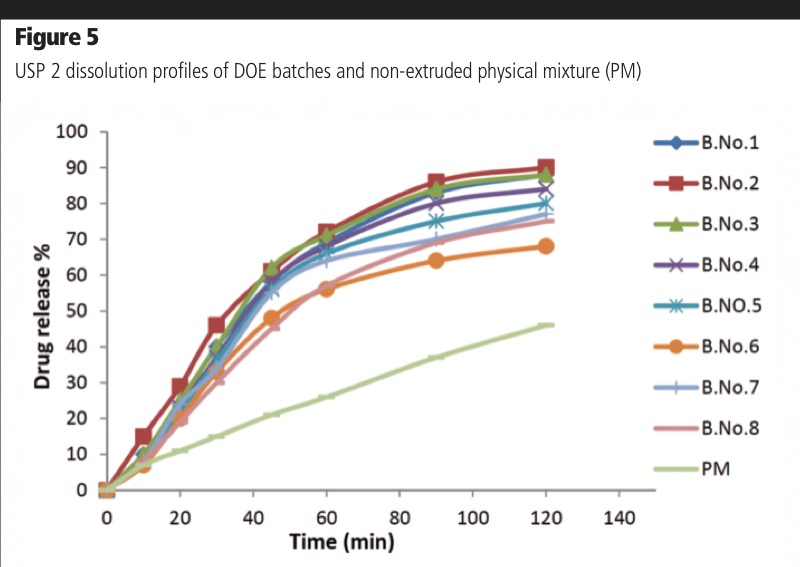
Indeed, f2 analysis confirmed the dissolution profiles batches 6 and 8 were dissimilar to batches 1–5 (f2 <50). Despite these differences, all ASDs displayed significantly faster dissolution compared with the physical mixture demonstrating the ability of the polymer to improve the dissolution rate of the poorly soluble compound.
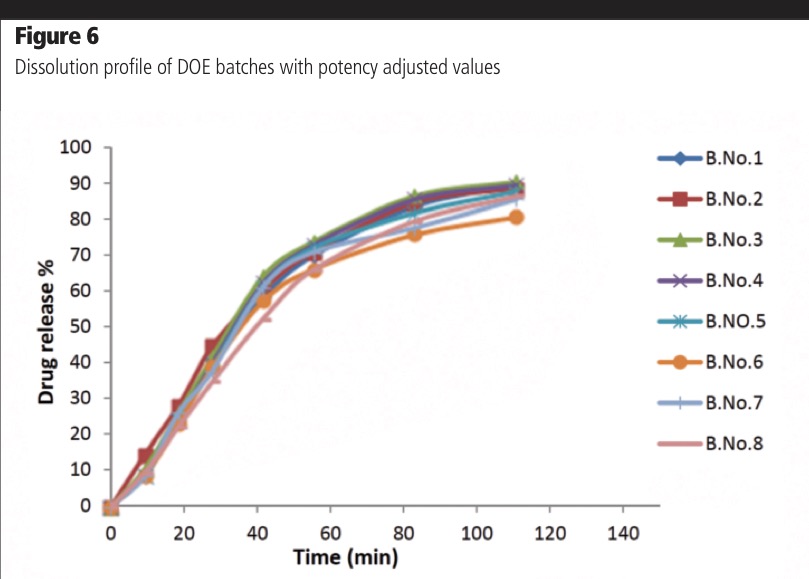
By adjusting for the analysed drug content of each sample, it is possible to understand if the dissolution rate is different among the batches because of changes in the polymer following HME. It can be seen (Figure 6) that when adjusting for drug degradation, the dissolution profiles for all process conditions are similar (f2 >50 for all comparisons). This confirms that the initially observed differences in dissolution were derived from changes in the API content, and not from changes in the polymer as a function of processing conditions.
Conclusions
The process conditions of temperature, screw speed and feed rate significantly impacted drug degradation for the poorly soluble compound ritonavir. The observed change in dissolution behaviour is a result of drug degradation and not changes to the polymer. The broad processing window of AFFINISOL HPMC HME allows exploration of the process parameter design space to study and minimise drug degradation for compounds with sensitivities such as RTV.
Reference
- S. Verma and V.S. Rudraraju, “A Systematic Approach to Design and Prepare Solid Dispersions of PoorlyWater-Soluble Drug,” AAPS PharmSciTech. 15(3), 641–657 (2014).
