Optimising the fitness of a manufacturing facility requires a multi-faceted approach, using in combination the three levers of lowering operating expenses, increasing throughput and reducing inventory.
Most companies today have taken steps to manage expenses and inventory levels without negatively affecting customer service. Companies have hired consultants to reorganise their production area to speed up the flow of goods by a few minutes here and there, or made significant investments in software to optimise production, resource and inventory allocations. Yet for many manufacturers it is still possible to cut additional days out of their production cycle by implementing a high-throughput, rapid microbial screening system.
Surprisingly, many of today’s modern, automated manufacturing facilities still test for microbiological contamination using a method dating back to the late 1800s. This traditional method relies on visual identification of microbial growth and averages five days for non-sterile and a minimum of 14 days for sterile products. During this wait time, raw materials, in-process batch or finished goods can be quarantined – adding days to manufacturing lead times, tying up working capital and ultimately leaving the facility far from fiscally fit.
Fortunately, current rapid microbial screening systems – which are validated and accepted by regulatory bodies – enable manufacturers to screen accurately for the absence of microbial contamination in only 24 hours; ensuring product quality and trimming four days from the average production cycle. Similarly, rapid methods can also be used for sterility testing; providing results in seven days versus 14-17 with traditional methods and cutting a week or more from production.
The financial savings can be tremendous. For example, one major pharmaceutical company was waiting 14 days for results from an in-process sterility test. During this past year, they validated and switched to a microbial screening system that enables them to release batches within two days, saving 12 days from their production cycle time and reducing this in-process wait time by 85%.
Table 1: Cost benefits of high-throughput, rapid microbial screening
|
Business opportunities |
Savings |
Typical benefit |
|
|
|
|
|
Reduce working capital requirements due
to shortened cycle times |
Decrease investment in product
manufacturing; fewer days to produce the same amount of product |
Free up cash for redeployment |
|
|
Reduce investment in inventory held in
quarantine |
Improved inventory turns |
|
|
|
|
|
Improve return on invested capital |
Reduce capital committed to ‘low return’
investments like quarantined inventory |
Improved return on working capital |
|
|
Redeploy capital to high return projects |
|
|
|
|
|
|
Save warehouse space |
Reduce number of days products are held
in storage |
Inventory adjustment |
|
|
Reduce costs for storing safety stock |
|
|
|
Overhead reduction |
|
|
|
|
|
|
Streamline the supply chain |
Release finished goods faster |
Increased inventory turns |
|
|
Respond to customer demand and
opportunities |
Shorten product time-to-revenue |
|
|
Accelerate revenue cycle |
|
|
|
|
|
|
Improve recovery from contamination
events |
Shorten recovery time by finding and
responding to problems sooner |
Improved customer service levels |
|
|
Reduce amount of scrapped product |
|
|
|
Replace lost product with clean product
faster |
Improved ability to identify problems and
react accordingly |
|
|
Minimise potential brand impact |
|
quantifying the value
Manufacturers can accurately quantify the financial value of implementing a rapid microbial screening system. For example, a high-throughput rapid system can be purchased and installed for less than b100,000, achieve payback in six to nine months, and realise an average five-year net present value in excess of b500,000 for non-sterile testing and well upwards of €1m for sterility testing at a single plant.
Likewise, implementing a rapid system goes a long way toward boosting a facility’s overall fitness score, with a host of quantifiable benefits:
Reduced working capital requirements – Rapid methods can decrease investment in product held in quarantined inventory at any given time. And since it takes fewer days to produce the same amount of product, the shortened cycle time also reduces safety stock requirements and its associated working capital investment.
Improved operational efficiencies – Shorter cycle times result in a leaner, more responsive manufacturing operation, which improves a company’s financial metrics – including return on invested capital and inventory turns.
Improved recovery from contamination events – Rapid detection means earlier detection of contamination when it does occur. This helps to minimise the amount of product affected, accelerate corrective action and get the facility back on track quickly. Also, risk of customer service interruptions or product recalls is minimised.
Saves warehouse space – Do facility space requirements factor in to your five-year planning horizon? How much physical space is required in your manufacturing and distribution facilities for in-process inventories and safety stock? Reduced inventories throughout your supply chain may decrease the need for outsourced warehousing, or defer the costs associated with facility expansion.
Shortened cash cycle – With rapid methods, finished goods are released to market faster, leading to an accelerated revenue cycle.
Swift decision making – Implementing a rapid screening system provides actionable information that allows product to move forward quickly. When contamination is detected, that batch can be isolated and attention focused on remediation and further process improvement.
As great as it is to take days out of the production cycle, the benefits more than double if there is a contamination event. That is because the sooner you know about a problem, the faster you can stop the line, get rid of the problem and produce – and test – a replacement batch.
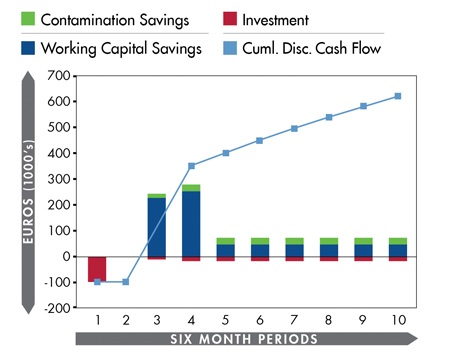
Potential savings accumulated during first five years following initial investment
In the end, a quicker response time minimises the overall economic impact of discarded or reprocessed goods and makes a company more responsive to customers, saving money and protecting the company’s reputation.
Even if you are managing a fairly efficient operation, most companies today have room for improvement. The realisable cost savings and reduction in lead times and working capital requirements will enable manufacturers to pursue more productive endeavours, such as funding new projects, developing new products or even conserving cash. Looking closely at their current product release methods will reveal if there is any time in micro-hold that can be reduced.
The old method from the 1800s may be comfortable, but it doesn’t do a thing for your figure(s).
You may also like
Trending
Articles
Upcoming
event
