Exosomes — a type of extracellular vesicle encased within a single outer membrane — have attracted the attention of several companies, medical centres, universities and research organisations in recent years.
The global exosomes market size was valued at $112.25 million in 2022 … and industry researchers suggest that the market will expand at a compound annual growth rate (CAGR) of 32.75% by 2030.1
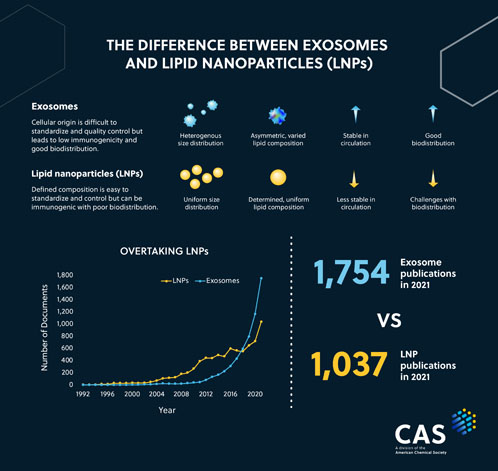
This trend is reflected in data from the CAS Content Collection, the largest human-curated collection of published scientific knowledge.2 In fact, there are currently more than 40,000 scientific publications (including journal articles and patents) related to exosomes, with the analyses revealing a steady and exponential growth.
In fact, in recent years, the number of publications related to exosomes has significantly surpassed that of LNPs, suggesting that there is growing interest in exosomes as a drug carrier system.3
Overcoming the limitations of LNPs
Synthetic drug nanocarriers such as LNPs have been used in mainstream drug delivery systems since the discovery of liposomes in the 1960s.4
Although LNPs have been instrumental to the success of the COVID-19 messenger RNA (mRNA) vaccines, their clinical application can be limited by several factors, including low bioavailability, toxicity, clearance from the bloodstream and the triggering of innate immune responses.5
Unlike liposomes, exosomes form naturally in living organisms. Exosomes are produced in the endosomes of most eukaryotic cells and are subsequently released in the extracellular space by fusion with the cellular membrane.

Although their function is still largely unknown, exosomes are key players in cell-to-cell communication, signal transduction and various other important physiological activities.6,7 Exosomes have also been linked to the pathogenesis of diseases such as cancer, as well as neurodegenerative and cardiovascular disorders among others.8–10
To understand how exosomes exert these functions, it’s important to consider how they are structured. Exosomes are the smallest of the extracellular vesicles released from cells, with a diameter of between approximately 30–150 nm.
Consisting of a lipid membrane and an inner aqueous medium, exosomes are small yet complex. In fact, nearly 100,000 proteins and more than 1000 lipids, along with a multitude of nucleic acids, have been linked to these diminutive membrane vesicles.11
It is this complex array of biomolecules that enables exosomes to survive and thrive upon release from the parent cell. Exosomes can circulate through a variety of biological fluids — such as blood, urine, saliva and cerebrospinal fluid —to reach their designated location.12
Owing of their unique attributes and significant impact on various physiological and pathological processes, exosomes have become increasingly prominent in the fields of drug delivery and diagnostics.
Compared with LNPs, they offer enhanced stability in circulation; their cellular lipid bilayer prevents enzymatic degradation and maintains the stability of the cargo.13 Because of their endogenous origins, exosomes have reduced immunogenicity compared with other delivery systems.
This means they are less likely to elicit an immune response or cause adverse reactions.14
Exosomes can transport their cargo over cellular hurdles, including the blood-brain (BBB) and other physiological barriers. Their ability to cross the BBB is particularly exciting as this biological barrier represents a significant challenge in drug delivery.
Owing to their small size and natural ability to transport cargo between cells, exosomes can deliver drugs to otherwise inaccessible sites, including the central nervous system (CNS).15 Because of their unique innate properties, exosomes are currently being explored in several applications, including drug delivery, therapeutics and diagnostics (Figure 1).
Figure 1: The difference between exosomes and lipid nanoparticles (LNPs)
The promise of exosomes
Drug delivery: Exosomes have shown promise as highly effective drug carriers. In addition to the properties discussed above, exosomes can be modified to target specific cell types or organs, thus enhancing their accumulation at the desired site of action.
This targeted delivery can minimise off-target effects and reduce the exposure of healthy tissues to the drug. Once at the site of action, the asymmetric lipid bilayer membrane of exosomes enables efficient fusion with the target cell membrane, facilitating the release of exosomes into the cytoplasm.16
Exosomes can also be engineered to encapsulate a wide range of therapeutic cargo, including small molecules, proteins, nucleic acids and even gene-editing tools.
Several companies are already exploring a range of exosomes for drug delivery, from neural exosomes for the treatment of stroke and neurodegenerative diseases to mucus-penetrating exosomes for respiratory diseases such as cystic fibrosis.17,18
Therapeutics: As well as being versatile and effective drug delivery vehicles, exosomes themselves also have therapeutic potential. Although research is currently at the preclinical stage, scientists are exploring exosomal microRNAs (miRNAs) for the treatment of cancer as well as neurodegenerative and cardiovascular diseases.19
Biomarkers: The properties of exosomes also contribute to their potential as valuable biomarkers and tools for disease detection, monitoring and personalised medicine. The bioactive substances present within exosomes have been shown to be altered during disease progression.
Therefore, exosomes can provide vital insights into the pathological status of the cells.20,21 As exosomes can be isolated from readily accessible biological fluids such as blood, urine and saliva, they allow for convenient, non-invasive sampling that minimises patient discomfort and enables more frequent monitoring.
Exosomes are also innately stable, so biomarkers carried by exosomes remain intact during circulation and storage, ensuring reliable detection and analysis.
Several preclinical companies and universities across the globe are researching exosomes for the diagnosis of various diseases, from tests for early cancer detection to a rapid isolation system that can potentially diagnose ocular disorders and systemic diseases from tear exosomes.22,23
Disease progression attenuation: Finally, as exosomes are involved in disease pathogenesis, a successful therapeutic strategy may involve reducing exosome production and circulation to normal levels to attenuate disease progression.
Several approaches are being explored to achieve this, from inhibiting exosome formation in the parent cells to inhibiting exosome uptake in recipient cells.24
Although initial investigations into these approaches show promise, gaining a comprehensive understanding of disease-specific mechanisms involving exosome pathways is crucial to identify targeted therapies mediated by exosomes.25
Exosomes: a bright future?
Exosomes have emerged as a contender to LNPs at prospective drug carriers, with unique properties that can enable them to overcome the limitations of synthetic drug delivery vehicles.
Yet, although exosomes hold great promise for drug delivery and diagnostics, several challenges still need to be addressed before their widespread use.
First, there is a need for improved isolation methods to obtain highly pure exosome products. From here, the standardisation of exosome preparation in compliance with good manufacturing practice (GMP) will need to be achieved before the widespread use and adoption of the technology.
Optimisation and improvement of exosome loading capacity and targeting is another prerequisite for large-scale application in clinic. Finally, the clinical applications of exosomes, although highly promising, are hindered by gaps in exosome knowledge.
Addressing these challenges through ongoing research, technological advancements and collaborations among researchers, clinicians and regulatory authorities will be instrumental in realising the full potential of exosomes in drug delivery and diagnostics.
References
- www.grandviewresearch.com/industry-analysis/exosomes-market.
- www.cas.org/about/cas-content.
- https://pubs.acs.org/doi/full/10.1021/acsnano.2c08774.
- https://pubs.acs.org/doi/10.1021/acsnano.1c04996.
- www.sciencedirect.com/science/article/abs/pii/S0168365914005434?via%3Dihub.
- www.nature.com/articles/nri2567.
- https://pubmed.ncbi.nlm.nih.gov/30076207/.
- www.nature.com/articles/s41392-020-00261-0.
- www.frontiersin.org/articles/10.3389/fnmol.2019.00240/full.
- www.frontiersin.org/articles/10.3389/fcvm.2021.767488/full.
- www.exocarta.org/.
- https://link.springer.com/article/10.1007/s13346-021-01026-0.
- www.americanpharmaceuticalreview.com/Featured-Articles/575432-Exosomes-The-Good-Bad-Ugly-and-Current-State/.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC5505007/.
- www.mdpi.com/2073-4409/9/4/851/htm.
- https://pubmed.ncbi.nlm.nih.gov/33429516/.
- www.arunabio.com/ab126intro.
- www.omnispirant.com/.
- www.frontiersin.org/articles/10.3389/fgene.2012.00056/full.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC4329112/.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC3281682/.
- https://mercybio.com/the-mercy-halo-test/.
- https://pubs.acs.org/doi/10.1021/acsnano.2c02531.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC3575529/.
- www.intechopen.com/chapters/80126.
