Taking a ‘design of experiments’ approach to process developments can increase product quality, thus creating value. Chris Claeboe, of Albemarle’s Fine Chemistry Services Division, uses two process validation examples to demonstrate how
The costs associated with batch failures can be significant. A process deviation or an out-of-specification product may require an incident corrective action report (ICAR) followed by a corrective and preventive action report (CAPA). While the root cause is being investigated, production ceases and time and resources may be diverted from other key activities, leading to lost revenue. In extreme cases, where repeated ICARs are required, a costly process re-validation may be needed.
Taking a design of experiments (DoE) approach to process validation can help to avoid these potential penalties and reduce the validation time. This tactic can increase the confidence that a process will be robust and repeatable, and has an accurate valuation; this level of confidence is one based on data rather than solely on the researcher’s experience.
It is no surprise, therefore, that companies such as Albemarle and the regulatory authorities have embraced DoE methods as tools for facilitating process validations using a quality-by-design initiative, as described in ICH Q8 (R2) – Pharmaceutical development (final), November 2009; and ICH, Process validation: General principles and practices (final), January 2011.
DoE methods
DoE is a statistical approach to experimentation in which variation is present and is usually applied to controlled experiments. DoE, or experimental design, is an established and proven methodology for product and process improvement in the pharma industry.
Traditionally, single-variant experimentation has been used to explore process design space, but varying one factor at a time can be relatively slow and expensive and, owing to a lack of statistical understanding, the interdependence of the factors is left largely undetermined.
In 1747 James Lind, while investigating scurvy, performed one of the first experiments to use positive controls. The use of controls might seem obvious to modern researchers, but Lind’s approach was revolutionary. Lind selected sailors suffering from scurvy but limited his sample to men who ‘were as similar as I could have them.’ Crucially, each of his six groups of five scurvy-affected sailors were given the same base diet, but with different supplements ranging from drops of sulphuric acid through cider to citrus fruits. Only the five men given two oranges and a lemon each day showed signs of improvement.
With just one variable determined as being the root cause of scurvy (a deficiency in vitamin C), Lind’s investigation was relatively simple. When three factors are considered, the scale of the task quickly escalates. In the schematic example below (Fig. 1), a cube represents a process design space with three variable factors (X, Y and Z). In this example, 180 experiments (4 x 4 x 5) could be required to fully explore the space if the factors were varied one at a time (Fig. 1a). It is also difficult to be sure which factor is causing a particular result, especially if one factor influences the impact of another.
In Fig. 1b, the same design space is explored using the central composite DoE approach. In this case, the whole process design space at once can be explored by varying each of the factors simultaneously. Only 18 or 21 experiments would typically be required highlighting the strength of the methodology (the central base experiment is repeated to reveal any systemic variation present in the baseline process).
The famous statistician and evolutionary biologist Sir Ronald Fisher pioneered the analysis of variance and elaborated modern DoE methods. He also wrote two seminal books on the subject: Statistical methods for research workers (1925), and The design of experiments (1935). One of his key contributions was to introduce randomisation into DoE methods, which is important as it reveals systemic bias variation and alleviates variations that may result from increased researcher efficiency.
For example, if an unstable high-performance liquid chromatography (HPLC) method is the primary analysis tool, the results of early experiments to establish the centre points may vary in comparison with later experiments because of the analysis method’s instability. Randomising the experiments helps to detect such sources of systemic variation.
Owen et al1 have provided an excellent contribution by elaborating on DoE procedures for process optimisation. They used a DoE method to optimise a desilylation reaction that produces alcohol. A narrow window of an acceptable yield (>93% alcohol) and impurity level (<2% lactose) was demonstrated with varying temperature and reaction time. In this case, the researchers were able to illustrate the critical process parameters and the control points that would facilitate the isolation of the desired alcohol according to the relevant quality attributes required.
A 16-step guide for optimising synthetic processes was presented; it should be noted that many of the steps are simple decision points such as deciding whether or not DoE methods are appropriate. The authors discuss DoE methods appropriate for pilot, screening, optimisation and robustness studies, each with increasing resource demands. In many cases, a central composite DoE approach is suitable for process optimisation studies.
trends in ICH guidelines
Customers rightly approach API process validation work using the ICH Q7 – Good Manufacturing Practice (final), November 2009 guidelines. However, the advantages of DoE approaches have initiated a change in philosophy that is reflected in new guidance documents (ICH, 2009 and 2011). As a company well known for solving complex chemical problems, Albemarle has been quick to embrace DoE methods and has incorporated the latest ICH guidelines into its normal procedures. This proactive approach has led to significant customer benefits, as demonstrated by the following case studies.
Case study 1: A small, virtual biopharma company wanted to validate and optimise its A + B → C (bimolecular) type reaction. A three-factor, 21-experiment, central-composite DoE method was initiated. A risk assessment following ICH Q9 – Quality risk management (final) June 2006 guidelines was performed.
Three factors that influenced the critical product-quality attributes were identified as:
- equivalents of B
- reaction concentration relative to solvent D
- reaction hold temperature
The isomeric impurity level, process yield and overall product purity were measured. The results gave an acceptable range for <5.75% isomeric impurity and >98% conversion (Fig. 2). Subsequent crystallisation purged the isomeric impurity levels of <5.75% to undetectable levels.
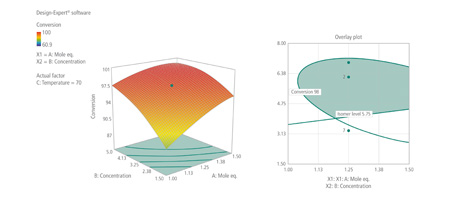
Figure 2: DoE results (right) and proven acceptable range (far right)
Higher reaction concentrations reduced the level of isomeric impurity formed. This was a highly significant result, as it entirely removed the next processing step, which would have been to distil out a large proportion of solvent before adding anti-solvent to initiate crystallisation.
The DoE approach demonstrated that the process could be run at pre-crystallisation concentrations, which significantly reduced the solvent raw material and distillation heating costs, and cut the process time. The simplific-ation also provided Albemarle with the opportunity to exert a greater level of process control during subsequent production campaigns and has been demonstrated to be consistent at commercial scales.
The DoE experimentation reduced the process validation time to about a month compared with an estimated minimum of six months for single variable experimentation and produced a robust and optimised process with raw material, energy and time savings.
Critically, there was no guarantee that traditional approaches would have led to the process being run at pre-crystallisation concentrations.
Case study 2: The same biopharma company wanted to validate and optimise
its bimolecular addition–elimination type reaction. A three-factor, 21-experiment, central-composite DoE method was initiated (Fig. 3). In this case, the identified factors that influenced the critical product-quality attributes were:
- the amount of input A
- the amount of input B
- the reaction concentration in solvent D
The HPLC assay purity and the HPLC and gas chromatography impurity levels were measured as responses to the process. The DoE approach demonstrated that a slight excess of input A gave optimum reaction conditions.
As for the previous example, the DoE approach facilitated a more thorough under-standing of the process design space than would have been achieved by following a more canonical one-factor-at-a-time approach. Although the process could have been validated using the industry’s standard methodology, the design space definition obtained using a DoE approach significantly reduced the risk of putative batch failure.
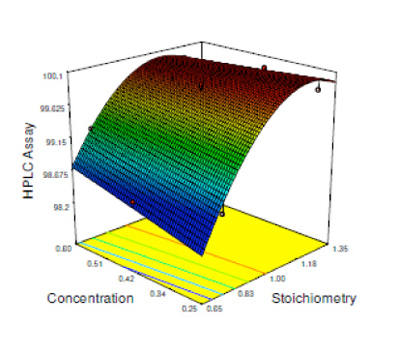
Figure 3: DoE results
confidence based on data
Although DoE methods are not a silver bullet, they can be valuable process validation tools. A DoE approach may speed up process validations and also help to ensure product quality through the front-end designing of a robust and repeatable process. Moreover, the confidence derived from DoE-based validation is founded on experimental and modelled data.
It is no surprise, therefore, that companies such as Albemarle, which look to provide maximum customer value, and the regulatory authorities have embraced DoE methods as an approach to quality by design. Ultimately, processes optimised using a DoE approach are likely to suffer from fewer batch failures, which protects revenues and reduces ICAR, CAPA and process re-evaluation costs.
references
1. Owen et al. Organic Process Research & Development, 2001, 5, 308–323
