Driven by the increased demand for healthcare, the global pharmaceutical market is projected to reach a value of $1.4 trillion by the end of 2027.1 As a result, laboratories will come under increased pressure to save on resources and develop smarter, more efficient manufacturing processes.
A precompetitive partnership between the Centre for Continuous Manufacturing and Advanced Crystallization (CMAC) at the University of Strathclyde and crystallisation equipment manufacturer, Technobis Crystallization Systems, resulted in an innovative DataFactory research project to autonomously develop a small-scale crystallisation system for active pharmaceutical ingredients (APIs).
In recent years, there have been many challenges in pharmaceutical manufacturing. The pandemic, for example, created unprecedented demand for vaccines, shut down many clinical trials, caused staff shortages and disrupted the manufacturing, transportation and supply of medicines across most of the world.
COVID-19 isn’t, however, the only factor causing supply chain disruptions. According to key players including Pfizer, the issue is multifactorial and affects the availability of APIs and other ingredients used in drug production.
These disruptors include natural disasters, energy crises and international trade tensions/conflicts, and cause factory closures, staff shortages and dramatic increases in the cost of APIs.2 This was demonstrated during the pandemic when the price of methylprednisolone acetate, a high-demand API for drugs treating COVID-19, rose from around $1000 to $2800 within a week.3
To meet demand, manufacturers must produce medicines quickly and in large quantities. Bringing a drug to market while minimising the time and resources required involves conducting many drug synthesis, crystallisation and formulation experiments, often with very little material. As a solution, companies are looking to Pharma 4.0 and digital technologies to facilitate quicker and continuous production.
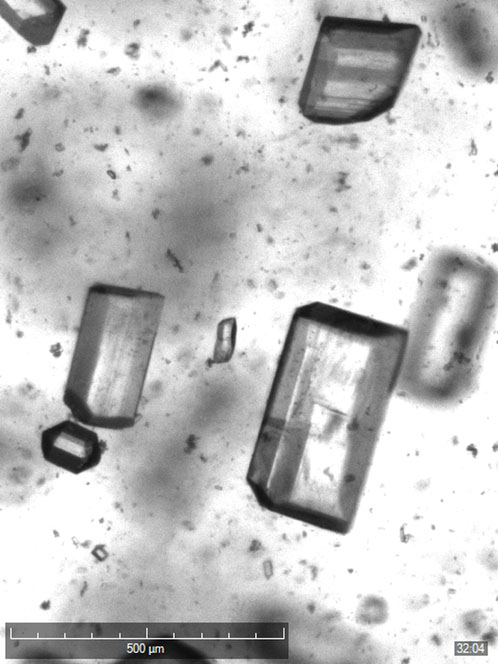
Real-world research
The CMAC team is developing a high throughput DataFactory of crystallisation experiments as a key part of the UK’s Engineering & Physical Sciences Research Council (EPSRC)-funded CMAC Future Manufacturing Research Hub.
This incorporates a collaborative laboratory robot controlled by machine-learning-based real-time decision-making software. The automated nature of the DataFactory experiments will allow the creation of a Findable, Accessible, Interoperable and Reusable (FAIR) database for API crystallisation.4
This database, in turn, will enable the development of machine learning models that can inform decision making in pharmaceutical manufacturing.
The team aims to use this DataFactory as part of its strategic research plan to implement a crystallisation classification system (CCS), which recommends downstream and manufacturing approaches. To achieve this goal, CMAC scientific and academic leaders introduced a pick and place robotic arm that can transport vials from one area of the laboratory setup to another.
One of the instruments the team needed was a benchtop product that could perform crystallisation experiments and analyse material simultaneously at millilitre volumes.
Generally, because particles in crystallisation experiments are small, chemists must remove samples from one apparatus and place them under various high-magnification and high-resolution spectrometers and microscopes to obtain crucial information about chemical characteristics.
However, transferring samples from one instrument to another means that crucial information regarding real-time crystallisation behaviour is lost.
“It is a tricky process to develop autonomous Pharma 4.0 manufacturing experiments that promote the efficient production of pharmaceutical products,” explains Dr Chantal Mustoe, research fellow at CMAC.
“This is largely because every new instrument in an autonomous setup adds another layer of complexity. We were looking for a single screening device on the market that could undertake crystallisation, solubility and both particle size and shape visualisation experiments, which led us to Technobis Crystallization Systems (TCS).”

“We felt sure that a collaboration would be beneficial, but we had a specific requirement to overcome multiple non-standard development challenges,” says Dr Mustoe. “For example, we needed the capacity to integrate other software and hardware platforms into the DataFactory in combination with the TCS technology. This meant that alterations to the company’s hardware and software were needed.”
Dr Mustoe continues: “We worked closely with a team of application and software engineers to combine what we do well — integrating digital design approaches with pharmaceutical manufacturing challenges — with TCS’s technology for solid-state research (Crystalline PV/RRs with AI-based image analysis and particle visualisation).”
“The Crystalline is an eight-reactor parallel crystalliser in which each reactor is independently controlled to generate data on particle distribution and shape,” explains Sarah Thompson, Sales Manager for UK, Ireland and the Nordics at Technobis Crystallization Systems.
“Each reactor has a camera positioned in a standard configuration that’s capable of up to two times magnification and a resolution of 1.9 microns per pixel. This allows scientists to visualise information on nucleation, growth and agglomeration at a micron scale. Finally, the system uses AI-based software to help the user reliably monitor their experiments.”
Data dilemmas
CMAC and TCS have been working together for the past 2 years to integrate two Crystalline instruments into the DataFactory at CMAC’s research facilities in Glasgow, UK. The project is still ongoing and a third instrument is set to be integrated into the process. One challenge the team faced in the early days was with the analysis software being closed to users.
“At first, the CMAC team couldn’t visualise any data being generated from the probes inside the multireactor vials. This was because access to the Crystalline software was limited to a defined set of users,” explains Thompson.
“After consulting with our software engineers, we were able to grant them access to their data repository and software instructions. This change, coupled with additional support from Technobis, is allowing the CMAC team to integrate the Crystalline software into an automated platform that remotely controls both the Crystalline and other instrumentation.”
“Furthermore,” says Dr Mustoe, “the software is helping CMAC to develop a crystallisation classification system (CCS). With the access made possible by Technobis, the AI-based software will be able to use the real-time data on solubility and crystallisation kinetics to recommend experimental approaches that might benefit the user."
"For example, using the data from previous crystallisation experiments, the software could recommend a different sample environment or heating profile for certain compounds to impact the attributes of the final product.”

Doing more with less
A strategic priority for CMAC is to design sustainable continuous processes for API and drug product research with minimal material, such as obtaining information about particle size and growth distribution.
These constraints ensure that they minimise the amount of material used while conducting the large number of experiments needed to gain information across a wide range of solvents and APIs. Fortunately, TCS has experience in designing versatile equipment for doing more experiments with smaller sample sizes and was able to offer the necessary support.
“For example,” says Thompson, “the latest version of Crystalline, which has been incorporated into the CMAC DataFactory, can operate with sample volumes of 2.5–5.0 mL. Meanwhile, other typical probe-based systems may need up to 100 mL to produce clear images for particle visualisation.
This, coupled with the superior particle viewing technology, makes visualising crystal size, shape and distribution possible at micron scale. Furthermore, the advanced inline particle viewing cameras and AI-based software help CMAC’s researchers to reliably monitor, design and optimise the crystallisation processes,” adds Thompson. Such efficiencies can reduce chemical waste and energy usage.
“For manufacturers, understanding the physical attributes of an API are critical to ensuring its behaviour in a drug therapy,” says Dr Mustoe.
“Therefore, the analytical capabilities of the Crystalline were a perfect fit for our DataFactory. Thanks to this research collaboration, our ongoing project to autonomously develop small-scale crystallisation systems for APIs is off to a great start."
"When engineering support is needed, we don’t have to wait for our quarterly call. The Technobis team is always accessible to discuss progress and are one step closer to finding a Pharma 4.0 solution for the efficient production of pharmaceutical products.”
References
- www.statista.com/outlook/hmo/pharmaceuticals/worldwide.
- www.pfizercentreone.com/insights-resources/expert-content/de-risking-api-intermediate-supply-crisis.
- www.gep.com/blog/mind/how-pharma-is-dealing-with-api-shortages?
- www.go-fair.org/fair-principles/.

