RNA interference, or RNAi, has become a hot topic in drug discovery in the past few years. Its importance was recognised with the award of the 2006 Nobel Prize to Craig Mello of the University of Massachusetts and Andrew Fire of Stanford University, who are credited with discovering gene silencing using double stranded RNA in the late 1990s, with their work on the nematode worm C. elegans. The technique has the potential for use in drug therapy because of its ability to stop the production of proteins that cause disease, and is one way in which the unravelling of the human genome is being exploited in medicine.
The human genome is the recipe book that the body uses to make proteins. To do this, a gene is first copied into mRNA – single stranded messenger RNA – and this is then translated into a protein as each set of three base codes for a specific amino acid. RNA interference is a natural process in which the mRNA is broken down before it can be translated into a protein. This process can be mimicked if a small double stranded piece of RNA that corresponds to a specific mRNA is introduced into a cell. The RNA-induced silencing complex, or RISC, within the cell recognises this fragment, splitting the two strands apart and keeping one strand within the complex. This then binds to and destroys the cellular mRNA target and production of the corresponding protein is blocked.
It is believed that it should be possible to create RNAi compounds that will interfere with the expression of any gene. This has the advantage that it would enable genes that are currently undrugable to be targeted, as long as an effective delivery mechanism could be developed. Two types of small RNA molecules are important in the RNAi process – small interfering RNA, or siRNA; and microRNA, or miRNA. They bind to longer strands of RNA to increase or decrease their activity, and both are being investigated as therapeutic options.
The company Alnylam Pharmaceuticals was set up to exploit the potential of siRNA, which are double stranded fragments of RNA, typically 20 to 25 base pairs in length, that interfere with the expression of specific genes. Alnylam recently announced it was starting a Phase IIb trial of ALN-RSV01 following positive results of a Phase IIa trial in respiratory syncytial virus (RSV), reported last year. The drug is being developed in collaboration with Cubist, and the trial was carried out in adult lung transplant patients infected with RSV. A total of 24 subjects were given the drug by inhalation or placebo once a day for three days. The trial achieved its primary objective of showing safety and tolerability of the drug over 30 days after treatment; there were no drug-related serious adverse events or discontinuations, and the side-effect profile was similar for the active and placebo groups.
Although the trial was designed to establish safety, there was evidence of improved recovery of lung function after 90 days. The Phase IIb study will see whether it gives a reduction in bronchiolitis obliterans syndrome, a life-threatening complication after lung transplants.
It is also working on RNAi-based therapies in a variety of different therapeutic areas. The furthest advanced of these is ALN-VSP, which is undergoing Phase I studies in the treatment of liver cancers, and preliminary data are expected later on this year. Several Phase I trials are planned for various other treatments, including one for transthyretrin mediated amyloidosis, and another for hypercholesterolaemia.
Silence Therapeutics has several potential therapies in development, three of which are now in the clinic for five different indications. The furthest advanced is PF-4523655, partnered with Pfizer and Quark, which is in Phase II for both diabetic macular oedema and age-related macular degeneration. The synthetic chemically modified 19 base pair siRNA was designed to inhibit the expression of the RTP801 gene, which was pinpointed by Quark in its gene discovery programme. It has been shown to enter cells in the retina, where it downregulates the target gene and, importantly, avoids activating toll-like receptor 3.
Another project in the clinic, this time Phase I, is QPI-1002, which it claims is the first systemically administered siRNA to reach human clinical trials. In one trial, its ability to prevent acute kidney injury after major cardiac surgery is being investigated, and the other is in preventing delayed graft function in kidney transplantation. The third siRNA in the clinic, again in Phase I, is an internal project, where Atu027 is being looked at as a potential treatment for cancers, including those of the gastrointestinal tract and the lung.
lowering cholesterol
Tekmira has two siRNA products in the clinic. The first is designed to treat hyper-cholesterolaemia. The common treatment for this condition – statins – are inhibitors of HMG-CoA, and can typically reduce LDL cholesterol levels by a half. Tekmira’s approach is to address the underlying cause of elevated cholesterol and target ApoB, a protein made in the liver that is involved in the assembly and secretion of very low density lipoprotein (VLDL), which is a precursor to LDL, and both of which are needed for the transport and metabolism of cholesterol. ApoB facilitates the lipoproteins’ uptake into the tissue, but it is thought it is undrugable by conventional means, and it has developed an siRNA to silence ApoB by knocking down the precursor mRNA that codes for ApoB protein in hepatocytes.
A Phase I study was completed in January of this year, and it was well tolerated overall with, importantly, no liver toxicity. The two subjects given the highest dose showed an average 21% reduction in ApoB, and 16% in LDL cholesterol, but because one experienced flu-like symptoms, a new Phase II trial is planned with a second generation siRNA.
A second target is PLK (polo-like kinase) as a potential cancer treatment. This protein is involved in tumour cell proliferation, and by inhibiting PLK, the tumour cell cannot complete its cell division and cell death results from cell cycle arrest. It has been shown to have potent antitumour effects in animal models, and there are plans for a Phase I study in relapsed or refractory cancer patients.
rxRNA approach
Another company set up to develop RNAi therapies and co-founded by Nobel winner Mello is RXi Pharma, which has called its approach rxRNA. Most RNAi compounds contain duplexes of two strands of chemically synthesised RNA, and their effectiveness depends on their length, the nucleotide sequence and the configuration of chemical modifications. RXi claims its rxRNA compounds contain many properties that will be required for RNAi compounds to make it into the clinic.
Depending on the target site, the company says, they can be up to 100 times more active than conventional siRNA, and more resistant to degradation by nucleases. They also have the potential to be more specific for the target gene and, they believe, more reliable at blocking immune side-effects than standard siRNA.
RXi has recently signed an agreement with another company, miRagen Therapeutics, to develop microRNA therapeutics. This collaboration will focus on the potential of RXi’s rxRNA technology against miRNA targets in the cardiac and neuromuscular disease areas.
miRNAs are short single strands of RNA, about 20 to 25 nucleotides in length, that regulate gene expression. They are transcribed from genes but, unlike mRNA, they do not encode proteins but instead prevent mRNA translation into proteins. These naturally occurring molecules can be modulated using synthetic oligonucleotides; these are usually either double stranded RNA fragments to mimic miRNA, or single strands as inhibitors.
Miragen’s most advanced product is in preclinical trials for post-myocardial infarction remodelling, where miRNAs involved in targeting cell proliferation and cell viability are being targeted. More recently, they have identified a miRNA that plays a crucial role in the progression of amyo-trophic lateral sclerosis or motor neurone disease.
In amyotrophic lateral sclerosis (ALS), the neuro-muscular synapse becomes incapable of transmitting the impulse that causes muscle contraction, and they believe they have found the miRNA that is crucial in this process. This may be a target for therapeutic intervention in this disease, for which there is currently no treatment available.
This isn’t the only joint venture set up by an RNAi company to look at miRNA: as well as its work on siRNA, Alnylam has set up a joint venture with Isis Pharmaceuticals, called Regulus Therapeutics, to discover and commercialise miRNA-based therapeutics. One area being worked on is diabetes, where blocking the Let-7 family of miRNAs appears to improve insulin resistance in a diet-induced obesity mouse model, and in normal mice it increased lean mass without affecting blood glucose. Other indications it is investigating include fibrosis, immunology and inflammation (in collaboration with GSK), and oncology.

Santaris Pharma is developing miRNA based products using its LNA platform
Another company that is working on miRNA, Denmark’s Santaris Pharma, now has RNAi products in the clinic. Its SPC3649 was the first drug targeting miRNA to be tested in humans when patients with hepatitis C were dosed in a Phase I study. Earlier results in four chimpanzees – the only non-human animal susceptible to HCV – which were published in December, looked promising.
The drug successfully inhibited miR-122, a microRNA that is expressed in the liver and is important for the virus to replicate. It reduced dramatically the levels of virus in both the liver and bloodstream of chimpanzees chronically infected with hepatitis C. They were given either 1 or 5mg/kg for 12 weeks, followed by a treatment free period of 17 weeks. The two given the higher dose had a decline in viral levels in the blood and the liver of about 2.5 orders of magnitude. Efficacy continued in the period after dosing, with no adverse events and no evidence of viral rebound or resistance. It is now undergoing Phase I trials in healthy volunteers.
The potential drug was developed using the company’s locked nucleic acid, or LNA, drug platform. It develops synthetically modified chemical versions of the normal nucleic acid building blocks of RNA, giving LNAs that improve the affinity for the target RNA. This boosts resistance to metabolism and improves tissue uptake, and means they can be delivered systemically to many different tissues, giving good potencies.
Regulus is also working on miR-122, and recently established a collaboration with GSK in the area. The plan is to identify a clinical development candidate later this year, with a view to filing an investigational new drug application at some point in 2011.
improving delivery
Delivery is going to be a particular problem for RNAi-based medicines. While small molecule drugs and large molecule biologics are generally pretty stable and can safely be carried around the bloodstream to their site of action, this is not the case for RNA-based drugs. They are degraded by a variety of nucleases in the blood and tissues, which renders them unstable in vivo.
This is not the only problem, however. They are rapidly excreted in the urine; tissue distribution is non-specific; and there is the potential for toxicities, including immunogenic effects and cytokine release. There is also the additional challenge of achieving cellular uptake and release once they reach the target cell.
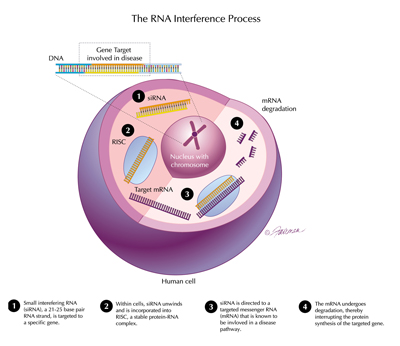
The RNAi process: 1. siRNA is targeted to a gene; 2. It unwinds and is incorporated into RISC; 3. It is directed to a targeted mRNA; 4. The mRNA undergoes degradation, interrupting protein synthesis of the targeted gene
This challenge is being addressed by several companies. Silence, for example, has developed a lipid delivery technology, which it calls AtuPlex. This uses proprietary lipid components, called AtuFect, that embed the siRNA molecules into multiple lipid bi-layer structures. The result is a nanoparticle structure, where the siRNA is combined with a cationic lipid, a fusiogenic lipid, and polyethylene glycol.
The company says it has shown it can increase systemic delivery to the endothelium, the liver and other tissues. It also gives better functional intracellular uptake of the siRNA compared with the siRNA alone, and circulation time and bioavailability are also improved. The particles can be freeze-dried, which means they will be easier to store and distribute.
These particles should be good for anti-angiogenic and antivascular therapies for treating solid cancers and cardiovascular disease. It has also developed nanoparticles that combine active siRNA molecules with its proprietary PolyTran biodegradable peptide-based polymers that should enable systemic administration of RNAi therapeutics, with a flexibility to incorporate siRNA sequences that act against a wide range of different disease targets. Both these nanoparticles and the AtuPlex particles can be modified by PEGylation to reduce immunogenicity, or by attaching targeting groups to help the siRNA reach the correct site in the body.
RXi, meanwhile, is working on direct delivery and systemic delivery, but has also developed a vehicle that will enable the RNAi to be delivered orally, rather than having to rely on injections. Its glucan encapsulated RNAi particles, or GeRPs, are designed to allow the RNAi to be formulated into pills. Mouse studies have shown that they can deliver the RNAi directly to macrophages, and this may pave the way for treatment of inflammatory diseases such as rheumatoid arthritis, bowel disease, psoriasis, and even diabetes and atherosclerosis.
encapsulation
GeRPS are hollow, porous micron-sized shells that can be filled with one or more RNAis. They are taken up by cells in the intestinal walls, and then transferred to immune cells in the gut-associated lymphatic tissue where they are taken up by macrophages. It is thought that the RNAis are then released into the cytoplasm, where they silence the target gene.
A different delivery technique is being developed by Tekmira. Snalp, or stable nucleic acid-lipid particles, are lipid nanoparticles that encapsulate nucleic acid molecules, including siRNAs, to enable systemic delivery to be achieved. According to the company, they have been shown to be effective in preclinical studies in delivering the drug to the target within the body, and also into cells, with systemic toxicity being minimised.
The technology is based on the enhanced permeability and retention effect. As the particles have a long circulation time in the blood, they accumulate where there are vascular leaks – notably at sites where tumour cells are growing or there is infection or inflammation. Once it has reached the site, the Snalp is taken up by the cells via endocytosis, delivering the siRNA to the cell.
For a technology unknown only 20 years ago – and unexplained until just over a decade ago – the potential of exploiting RNAi in drug discovery has come on dramatically in the past few years. There is still way to go before an RNAi-based therapy is available on the market, and a variety of challenges need to be overcome, such as effective delivery.
But it has the potential to provide therapies for diseases and targets that cannot be addressed by the current paradigm of small molecules and large biologics.
