Although the issue of poor solubility is not new, the increased use of drug discovery techniques such as combinatorial chemistry and high throughput screening have led to a growing number of compounds entering the development pipeline that have low solubility in both water and organic solvents.
Referred to as ‘’brick dust’’ compounds, these candidates require expertise in methodologies that can help to overcome these challenges.
Using amorphous solid dispersions to overcome solubility issues
Low solubility has plagued drug discovery for decades, with 70–80% of the current development pipeline consisting of poorly soluble molecules.1
Better solubility is, therefore, regarded as a key driver to improve bioavailability and efficacy.2 Several formulation strategies can be used to improve solubility, with conversion to amorphous solid dispersion (ASD) being the most frequently used strategy from 2000 to 2020 (Figure 1).
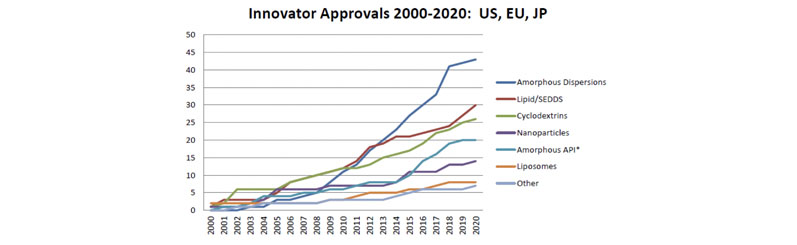
Figure 1: Cumulative enabling solubilisation techniques used on marketed products from 2000–20202
The most commonly used technique to manufacture ASD systems is spraying drying; its fast-drying rate enables the drug to be kinetically trapped in the amorphous form.3
The spray drying process (Figure 2) begins with a solvent, the active pharmaceutical ingredient (API) and a concentration-enhancing polymer in a solution tank.
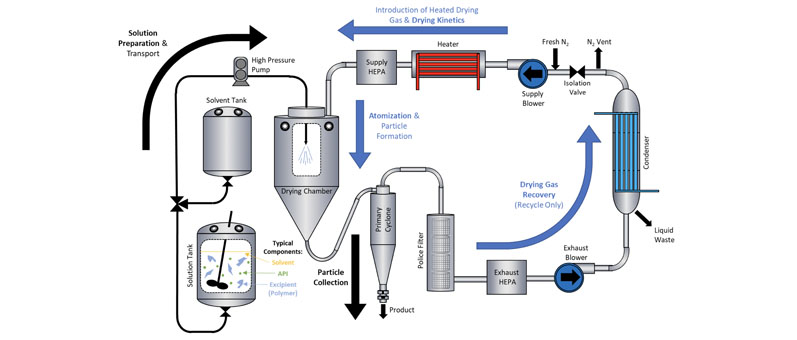
Figure 2: Spray drying process overview
The solution is then pumped at high pressure through a nozzle — typically a pressure swirl nozzle — into the top of the spray dryer. This sprays droplets of the solution into a heated gas, usually nitrogen, forming solid particles that are trapped in the amorphous phase.
The nitrogen gas and solid particles then flow into a cyclone, where the amorphous solid dispersion is collected. Finally, the solvent is condensed and the nitrogen is recycled back to the process.
For this technique to be successful, all of the components within the solution tank must fully dissolve during the spray drying process. However, brick dust compounds with poor solubility in both water and organic solvents can make this especially challenging.
Low organic solubility, in particular, can lead to small particles that can negatively impact downstream processing. It can also make the process uneconomic at a commercial scale.
Some manufacturers overcome this by selecting solvents in which the compounds are more soluble, such as dichloromethane (DCM) or tetrahydrofuran (THF). However, these solvents are also more toxic.

Furthermore, DCM presents significant environmental risks, resulting in emissions that are increasingly regulated, thus limiting production.
With THF, there is the risk of peroxide formation, which can cause chemical degradation in a product and increase the risk of explosion. Both of these solvents are potentially incompatible with certain equipment.
Although some programmes at Lonza do rely on these solvents, we are looking to move away from them altogether as part of our sustainability goals. We have developed alternate methods that allow poorly soluble compounds to dissolve in more acceptable solvents for safety and exposure reasons.
These techniques include thermal-shift methods, wherein the solvent is either warmed in the tank prior to spray drying or heated using a heat exchanger to supercritical temperatures prior to pumping into the spray chamber.4 Both methods allow for increased compound solubility in more favoured solvents.
Alternatively, when the incoming drug substance is either a weak base or a weak acid, volatile processing aids can be used to include the compound’s solubility. In the case of a weak base, acetic acid can be used to form an in-situ salt.5
During the spray drying process, the acetic acid is volatilised, resulting in the free base compound being reformed in the final amorphous dispersion.
Similarly, if the incoming drug substance is a weak acid, ammonia (added as ammonium hydroxide) can be used to increase the compound’s solubility in the solvent.
The ammonia is also removed during spray drying, this time reforming the free acid drug substance. Each of these methods has been used to improve the throughput of the spray drying process without having to resort to less desirable solvents.
In conclusion, spray drying is a well-established process that is used to make multiple commercial products as it can make ASDs to solubilise poorly soluble compounds.
However, as the API chemical space evolves — as evidenced by the growing numbers of brick dust compounds — it is necessary to continually innovate to maximise process efficiency and sustainability.
References
- www.americanpharmaceuticalreview.com/Featured-Articles/573402-Poor-Solubility-Where-Do-We-Stand-25-Years-after-the-Rule-of-Five/.
- https://doi.org/10.3390/pharmaceutics13101682.
- https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.0c00798.
- D.T. Friesen, et al., “High Temperature Spray Drying Process and Apparatus,” US Patent No. 9248584B2 (2016).
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8950584/.

