Solid dispersion technology is an effective technique for increasing bioavailability of poorly soluble APIs. Timothy Bee, of International Specialty Products, and Nicole Neub, of Coperion, review the use of hot melt extrusion as a means of direct mixing of materials to form solid dispersions
It has been estimated that 40–60% of drugs in development have poor bioavailability due to low aqueous solubility. This percentage is likely to increase in the future with the increased use of combinatorial chemistry in drug discovery targeting lipophilic receptors.
Poor bioavailability results in increased development times, decreased efficacy, increased inter- and intra-patient variability and side-effects, and higher dosages that reduce patient compliance and increase cost. Thus, the ability to improve drug solubility and hence bioavailability through formulation and process technology is critical to improving a drug product’s efficacy and safety and reducing its cost.
Solid dispersion technology, where the API is dispersed at the molecular or nanoparticle level as an amorphous material within a solid matrix, is a proven and highly effective technique for improving drug solubility. Solid dispersions are most practically and most commonly produced at the lab through commercial scales via either melt extrusion or spray drying process technology.
Each technology has its advantages and limitations. A previous article (Manufacturing Chemist, March 2010, pp24–25) described the advantages and limitations of spray drying and the critical process parameters with respect to preparing solid dispersions. This article provides a brief review of solid dispersion technology and describes the specific process inputs for melt extrusion technology that must be considered when preparing solid dispersions.
Solid dispersions are molecular (thermo-dynamically stable solid solutions) and/or colloidal (kinetically stable solid suspensions) dispersions of the amorphous active pharma-ceutical ingredient (API) dispersed in a polymeric matrix. As a result of their morphology and their thermodynamic and thermomechanical properties, solid dispersions increase drug surface area, reduce drug crystallinity and stabilise the system during storage and in vivo to inhibit drug re-crystallisation. When properly formulated and processed, the result is a system with excellent shelf-life stability and dramatically enhanced drug solubility and bioavailability that can be orders of magnitude greater than that of the purely crystalline drug form.
While formulations of solid dispersions have been known since the 1960s to improve drug bioavailability, the technology began to receive much greater attention in the late 1990s as a means to address the development challenges of the increasing number of poorly soluble APIs coming out of drug discovery research. The use of the technology has become widespread over the past decade with the adaptation from other industries of common process technologies like hot melt extrusion and solvent spray drying that made solid dispersions practical from laboratory feasibility through to commercial production.
Melt extrusion is commonly used to prepare solid dispersions by:
-
1. softening the polymer and any desired adjuvants in a twin screw extruder
2. adding the API to the molten mixture and mixing it into the system as it flows through the extruder
3. rapidly cooling the extrudate to form strands of polymeric glass with embedded API and
4. milling the glass strands into a powder suitable for subsequent finished formulation.
As it is a continuous process and does not involve the use of extraneous ingredients like solvents, melt extrusion is a cost efficient, effective system for forming solid dispersions. However, while melt extrusion is a highly efficient mixing technology, it does not achieve the same level of mixing as spray drying for some APIs. In addition, particular caution must be taken with heat-sensitive APIs through proper process control and equipment design. The key features and process considerations for melt extrusion technology applied to solid dispersion production are described below.
melt process technology
Melt extrusion is the most efficient and economical process to melt and mix solid materials directly. While melt extrusion has been employed in other industries for more than 50 years, the technology did not begin to receive attention in the pharma industry until the mid-1990s. In pharmaceutical applications, melt extrusion has been used for granulation, taste masking, solubility and bioavailability enhancement, sustained release products and polymeric implants. Product throughput, mixing efficiency and temperature exposure can be controlled through the proper selection of melt extrusion equipment and process parameters, including feed rates, temperature zone settings and screw element configuration.
Furthermore, the hot melt extrusion process and hot melt extrusion equipment are readily scalable, from lab- to pilot plant-scale, through to commercial production.
In the hot melt extrusion process, the formulation is processed above the glass transition temperature of the polymer/API/ adjuvant system to mix the active ingredient with the polymer on a molecular level. When carried out in a twin screw extruder, this continuous process offers numerous advantages over other melt processing methods, including:
- a short residence time at elevated temperatures (i.e. minimal thermal stress on heat sensitive APIs)
- a high reproducibility
- intensive mixing (dispersive or distributive mixing)
- high throughput rates
- a self-wiping effect from the closely inter-meshing screws
Because of their excellent mixing behaviour and degassing possibilities, co-rotating twin-screws are particularly suitable for hot melt extrusion. In addition, the modular concept of the individual screw elements and use of different heating zones along the process barrel allows individual adaptation of the processing section to different product and formulation requirements. Individual steps, such as melting, dispersive mixing, distributive mixing, degassing and pressure build-up, can be controlled very selectively and very effectively.
The key factor in preparing solid dispersions through melt extrusion is the use of an individually developed screw configuration and zone temperature profile for optimal intake of individual formulation components (polymer, API, etc.), melting the polymer, mixing in the API, removal of any volatiles and discharging the end product.
It is mandatory that each zone has its own heating and cooling circuit and that the temperature adjustment of the individual process steps be well-controlled.
The main task in pharmaceutical hot melt extrusion is to control the process such that the required softening temperature of the polymer is exceeded, but the degradation temperature of the API is not reached. In the case of semi-crystalline polymers, the temperature of the melting zone is normally set a few degrees higher than the melting point. In the case of amorphous polymers, the temperature of the melting zone should be above the polymer glass transition temperature.
For many APIs, the delta in temperature between the softening point of the polymer and the degradation temperature of the API is very narrow and therefore offers only a very small operating window. Thus, tight process control is critical.

Figure 3: Intermeshing profile of the twin screws
Source: Coperion GmbH
Key factors to control the process within these tight limits are the heating and cooling device in the process zone where the API is dispersed into the polymer. Modern extruders provide the ability to cool the barrel in the mixing zone where high levels of shear can contribute to local heating effects. Over the entire process section a combination of dispersive or distributive kneading or mixing elements as well as back-conveying elements are used to mix the API into the polymer. These elements also create shear energy that can increase the product temperature. In combination with the heating and cooling device of each individual barrel section and relaxation zones between the high-shear areas, a gentle and sensitive temperature profile can be achieved.
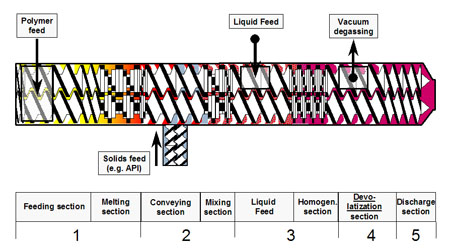
Figure 4: Diagram of the individual process steps in a Coperion twin-screw extruder
Source: Coperion GmbH
Feeding & melting zone (1): In the first process zone (feeding zone) the polymer, and any required adjuvants, are fed into the extruder via a gravimetric solid feeder. In the melting zone, the polymer is melted by the use of dispersive and distributive screw elements.
Depending on the product specifications it can be beneficial to feed a premix of raw materials into the upstream feeding zone.
Incorporation of additional components such as APIs (2&3): Downstream of the melting section, additional solids (depending on the product requirements), liquids or the API are added. In some cases, it can be beneficial to add the API nearly at the end of the process section to decrease the thermal stress on the API. Although the residence time of the material in the melt extruder is already very short, adding the API downstream in the process section can reduce the API residence time (and thermal exposure) to an absolute minimum.
Devolatilisation zone (4): Just before the end of the process section the product is devolatilised. Due to the axially open screw channels in the co-rotating twin screw extruder, the devolatilisation zone must be sealed by completely filled zones on either side of the devolatilisation opening to prevent, for example, extraction of components that have not yet been incorporated from upstream zones. Back-conveying (left handed) screw elements are used to create a melt filled zone. The conveying elements in the devolatilisation zone are designed to operate partially filled to provide as large a product surface as possible for devolatilisation and also to prevent product discharging through the vent.
Discharge section (5): The pressure build-up zone is located at the end of the extruder up-stream of the die or other integrated downstream equipment. The pressure consumed by the up-coming subsequent unit (e.g. die face pelletiser) has to be generated in the discharge zone.
downstream section
When the product leaves the process section of the extruder there are multiple options for shaping and cooling the extrudate. Different dies like strand dies, flat dies or co-extrusion dies are available to shape the melt before it solidifies. Downstream processing includes equipment like die face pelletisers, strand pelletisers, cooling calanders and forming calanders.
In summary, solid dispersions have become an established formulation technology for improving solubility and hence bioavailability of poorly soluble APIs. Solid dispersions are effectively prepared and commercialised by solvent spray drying and melt extrusion. In melt extrusion, a number of formulation, equipment and process factors influence the performance and production of the resulting product including polymer selection, polymer to API ratio, screw configuration and temperature profile along the process section.
