Among various manufacturing processes, lyophilization plays a crucial role in preserving delicate biopharmaceuticals. As companies adapt to the new GMP guidelines, implementing aseptic process simulation (APS) in lyophilisation has become both crucial and tricky.
This article dives into the complexities of APS for lyophilisation, highlighting the challenges manufacturers face in meeting the updated regulations and providing recommendation for a better practise.
The challenges in APS for lyophilisation
Designing an APS that accurately mirrors the complexities of lyophilisation is a formidable task. According to paragraph 9.33 of Annex 1, “the APS should imitate as closely as possible the routine aseptic manufacturing process and include all the critical manufacturing steps”. Meanwhile, the process that may affect the viability or recovery of contaminants should be avoided.
A common lyophilisation cycle consists of 3 steps: freezing, primary drying and secondary drying. During freezing, the product is cooled to at least -40 °C to achieve complete solidification of the product.
In primary drying, the drying chamber will be evacuated to a certain pressure, normally in range between 0.02 and 1 mbar. In the meantime, the shelf temperature is increased to provide the energy for sublimation which allows the solvent to be converted from solid into gaseous form directly.
In the secondary drying, the shelf temperature is further increased, generally between 10 °C to 40 °C, so that the unfrozen solvent could be removed by desorption and diffusion. A common lyophilisation cycle could take 2 to 7 days.
Owing to the process condition of the lyophilisation, the viability and recovery of contaminants could be affected by three main factors:
- Temperature: when subjected to freezing temperatures, the media undergoes solidification, diminishing the viability and recovery of contaminants. Conversely, elevated temperatures during secondary drying may also adversely impact the viability and recovery of contaminants. It is noteworthy that the recommended storage temperature for nutrient media ranges from 2 to 25°C.
- Pressure: maintaining the media temperature within the recommended range of 2 to 25°C is crucial. However, applying pressure within the range of 0.02 to 1 mbar can induce water boiling within the media, thereby affecting the recovery of contaminants.
- Duration of the cycle: even with pressure and temperature kept within the desired range, an extended duration of a lyophilisation cycle may jeopardise the viability and recovery of contaminants. This risk arises from the loss of water in the media through evaporation.
- Condition for stoppering: the stoppering condition in a regular lyophilisation process could negatively affect the viability and recovery of contaminants since the growth of most of the microbial in media requires oxygen while the vials are generally stoppered under vacuum or with backfilled inert gas.
Points to consider while developing an APS plan for lyophilisation
All key considerations in developing an APS plan are listed in paragraph 9.36. As discussed above, it's challenging to develop an APS plan that fully represents a typical lyophilisation unit operation (Figure 1).
During the operational phases of loading and unloading, chamber evacuation, and aeration, the APS settings deviate from those of a standard lyophilisation process, as explained earlier.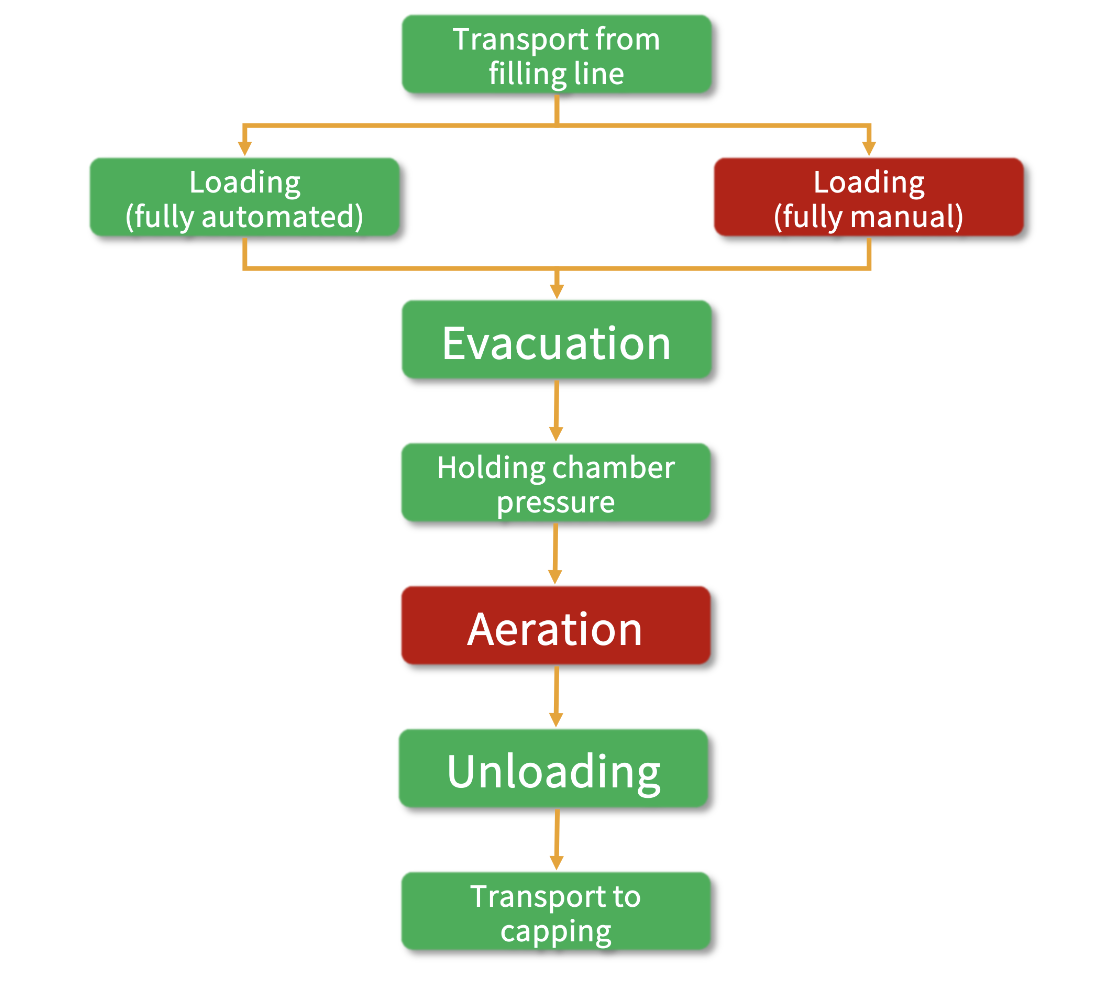
Figure 1: The lyophilisation operation
Loading and unloading
Fully automatic loading: Since the half-stoppered vials are operated under Grade A clean room condition without human intervention, the risk of contamination is low.
Recommendation: Only fully load the last shelf with media-filled container and the rest of the shelves with stoppered empty containers.
Fully manual loading
In contrast to fully automatic loading, fully manual loading introduces operator intervention, making it a high-risk activity. In accordance with Annex 1, maximum load must be simulated in the APS.
Recommendation: Perform maximum loading with media-filled containers and empty containers.
Shelf temperature settings
To safeguard the viability and recovery of microbes, it is essential to steer clear of extremely low or high shelf temperatures, as the recommended storage temperature for nutrient media falls within the range of 2 to 25 °C.
Recommendation: When loading is conducted at ambient temperature, the shelf temperature in the APS can be straightforwardly adjusted to the standard room temperature of the aseptic area. If loading is carried out using a pre-cooled shelf, the shelf temperature in the APS can be configured to 5 °C.
Chamber pressure holding phase
Pressure setting: As previously clarified, maintaining the pressure within the typical lyophilisation range could lead to media boiling and should, therefore, be avoided.
Recommendation: Partial vacuum in range between 800 and 900 mbar can be utilized in this phase.
Duration of the pressure holding phase
Because the pressure setting in APS falls within the range of 800 to 900 mbar, the airflow during the chamber pressure holding phase in APS differs from that of a standard lyophilisation process for two specific reasons (Figure 3):
- Vapor flow resulted from sublimation: During the primary drying phase in a typical lyophilisation cycle, the sublimation of the solvent typically generates vigorous vapor movement. In instances where the chamber is not adequately sterilized, this turbulence could potentially transport contaminate from the chamber into the container. In the APS, sublimation does not occur. Instead, vapor arises solely from the gradual evaporation of water in the media, which is considerably weaker compared to sublimation. This substantially reduces the risk of contaminants entering the containers.
- Airflow resulted from gas bleeding aiming pressure control: During the primary and secondary drying phases of a lyophilisation cycle, a minimal quantity of inert gas or sterile air is introduced into the chamber through the gas bleed valve to maintain a constant pressure at the setpoint. This introduces additional turbulence, potentially elevating the risk of contamination within the containers. In the APS, the pressure is configured between 800 to 900 mbar, well exceeding the operational range of the gas bleed valve. Consequently, there is no airflow from gas bleeding or the associated turbulence. This effectively reduces the risk of contamination within the containers.
- Difference between chamber and ambient pressure: In the primary and secondary drying phases of a typical lyophilisation cycle, the substantial pressure difference between the chamber and the ambient environment poses a high risk of air leakage. This implies a heightened risk that contaminants could be transported from the ambient surroundings into the containers.
Conversely, in the APS, the disparity between chamber pressure and ambient pressure is significantly smaller, resulting in a considerably reduced risk of contamination due to air leakage.
Because of the variation in airflow during the sublimation/pressure holding phase between lyophilisation and APS, the duration of the pressure holding phase is no longer a critical factor. Additionally, prolonging the pressure holding phase could potentially lead to media damage through the evaporation of water.
Recommendation: Hold chamber pressure for 10–12 hours.
Aeration
Condition for stoppering: In lyophilisation, except for tray-based processes, containers are fully stoppered before aeration, whether under vacuum or with backfilled inert gas. If containers filled with media are stoppered under vacuum or with backfilled inert gas in the APS, there will be a significant and dramatic reduction in the viability and recovery of the microbial.
Recommendation: Containers should be stoppered after aeration with sterile air.
Design of aeration cycle: In a typical lyophilisation cycle, the substantial pressure difference between the chamber and the ambient environment during aeration could lead to violent turbulence and long duration of aeration, posing a high risk of contamination.
However, in standard lyophilisation operations, except for tray-based lyophilisation, containers are fully stoppered before aeration. This practice significantly reduces the risk of contamination, nearly eliminating it.
In the APS, the pressure difference between the inside and outside of the chamber is considerably lower during aeration, and the duration is shorter, indicating a reduced risk of contamination. However, it is crucial to note that containers are not fully stoppered at this stage.
Recommendation: Perform chamber aeration using sterile air from partial vacuum until reaching atmospheric pressure and maintain this pressure for 1 hour to facilitate particle settling. To replicate the "worst-case scenario," the chamber may be evacuated to partial vacuum, specifically 800 – 900 mbar, repeated twice in this cycle. Ultimately, containers are stoppered at atmospheric pressure. 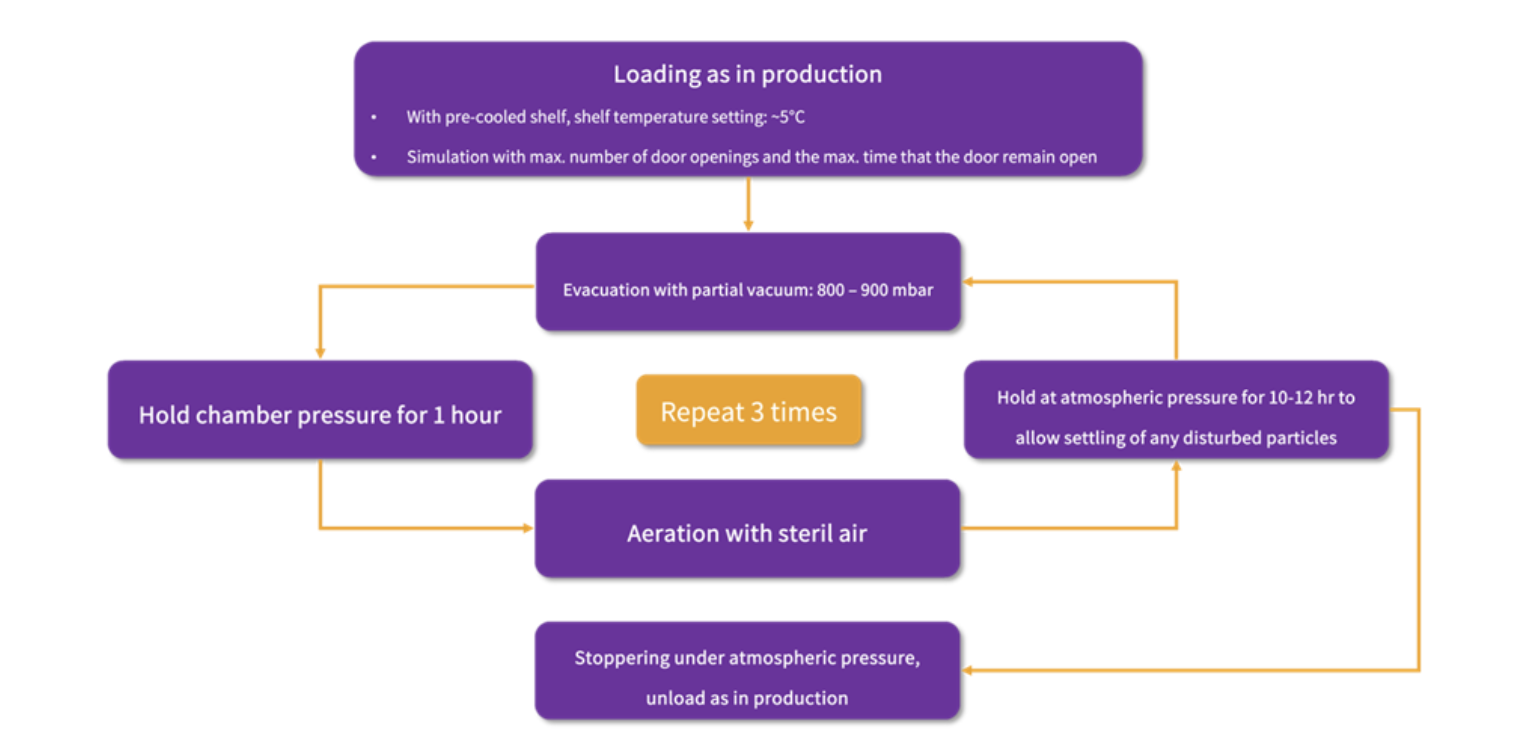
Figure 2: A complete APS plan for the lyophilisation process
In conclusion, aligning with the lyophilisation process conditions and the stipulations outlined in EU GMP Annex 1 poses a challenge for achieving an APS that perfectly replicates the entire lyophilization process.
However, by adapting certain aspects of the process, it is possible to devise an APS plan that closely mimics routine aseptic lyophilisation while ensuring the viability and recovery of microbes.
Annex 1 provides additional details on conducting APS, including considerations like media volume and container size, which are not covered in this article due to length constraints.
References
- David Hamilton, et al., 2021, A Better Approach To Aseptic Process Simulation For Lyophilized Products, Guest Column, Outsourced Pharma
- Varadharaj Vijayakumar, 2021, A Regulatory Perspective: Rationale for Hold duration of Partially Stoppered Media Filled Vials, Lyophlilization World
- Haseley, P. and Oetjen, G.W., 2018, Freeze Drying, 3rd edition, Wiley-VCH

