Abstract
Low-dose drug formulations contain highly potent active ingredients with high bioavailability and a narrow therapeutic window, posing significant challenges in development, scale-up, quality control, and analytical methods. This study assesses mixing uniformity in the pre-mixing stage of the HSG-12 high shear granulator using a binary mixture of metformin and lactose as a model. Metformin, the active pharmaceutical ingredient (API), comprises one-thousandth of the mixture, with lactose serving as an excipient to simulate mixing uniformity in low-dose drugs. Testing demonstrated excellent final relative standard deviation (RSD) of mixing uniformity at 1.28%.
Introduction
Low dose drug products, defined as those containing a single dose of no more than 1 mg, present significant complexities in their manufacturing process, and quality control, and demonstrate high in-vivo activity. These products are characterized by extremely low unit dose content, a high excipient/API ratio (up to 500 to 50,000), and are prone to loss and contamination during manufacturing. Achieving good recovery rates requires highly sensitive analytical methods and precise pre-treatment techniques. Low dose drugs often fall under BCS Class I or III categories, necessitating stringent requirements for content uniformity and product stability during both production and storage. [1]
Content Uniformity
Content uniformity is a critical quality attribute as it directly impacts therapeutic efficacy and poses safety risks for drugs with narrow therapeutic windows. Factors influencing content uniformity include:
-
Materials: Particle size, distribution, shape, roughness, bulk density, static charge, moisture content, friability, flowability, etc.
-
Process: Proportion of material volume to container volume, material volume to mixer volume, mixing time, mixing speed, etc.
-
Equipment: Significant performance variations exist between different equipment.
Wet Granulating
Wet granulating enhances the content uniformity of low-dose drugs, prevents component separation, increases the dissolution rate of hydrophobic drugs, and reduces dust generation, thereby minimizing cross-contamination and air exposure. [2] Wet granulators are widely utilized for mixing low-dose drugs due to their high efficiency in achieving homogeneous blends.
Pre-mixing is the first step of the high shear wet granulating process. In the pre-mixing stage, the materials containing powders are mixed uniformly. Typical pre-mixing time can be chosen in the range of 2 to 20 minutes [3] depending on the mixing size. Given constant ingredient particle size, material density and mixing conditions (rotating speed and duration), the performance of the wet granulator is a major factor influencing the mixing uniformity. The advanced design of the wet granulator bowl and granulating elements plays a critical role in ensuring efficient mixing.
Bowl design: Bowl shapes vary among manufacturers, with considerations including the ratio of straight to conical sections and the design of the top lid. The arc design of the top lid aligns with the trajectory of material movement (see Figure 1), minimizing residue and optimizing the mixing and granulation process.
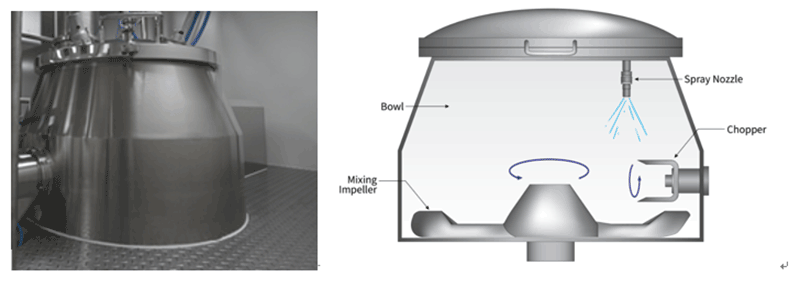
Figure 1: Bowl geometry of wet granulation
Mixing impeller design: The impeller's shape, angle and elevation directly influence the horizontal and vertical movements of the materials. The design combined with the bowl’s straight section, determines the retention time in the high-pressure zone. As shown in the mixing impellers from the internationally renowned wet granulator suppliers, tangential impellers are now mostly used in the high shear wet granulators. (Figure 2)
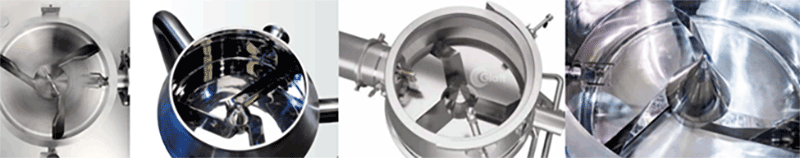
Figure 2: Mixing impellers from other internationally renowned suppliers
The tangential impeller used in this study can avoid the mixing dead points formed by the impeller and main shaft. Special elevation design can reduce the speed difference between the materials at different heights. There is a palm-side design at the end of the blade to enhance the propulsion of the impeller. The mixer has a conical design to reduce the residues of the mixed materials. (Figure 3)

Figure 3: Mixing impeller of wet granulator
Chopper design: The bottom-driven high shear wet granulator typically uses a side-driven chopper to provide shearing force during the solution adding and wetting to break up the lumps of soft materials. Low-speed shearing, coordinated with the impeller speed, prevents the formation of dead zones and limits the impact on particle size. For different types of choppers, it is also necessary to examine the rotating speed at different granulating stages. In this study, one U-shaped chopper is used (Figure 4, a).
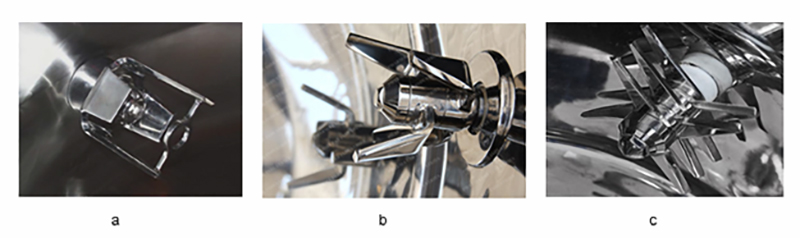
Figure 4: Different types of choppers
This article exemplifies the influence of the wet granulator design on mixing uniformity through simulated testing of low dose drug mixing. Specifically, API distribution during the pre-mixing stage was systematically studied based on a series of combinations of model drug formulations. The model drug formulation consists of metformin (API) and lactose (excipient).
Material and method
Material
A two-component model drug formulation was utilized in this study, consisting of metformin as the Active Pharmaceutical Ingredient (API) and lactose as the excipient. The pre-mixing was conducted using a High Shear Granulator (AUSTAR, HSG-12).
Mixing
The feeding amount of metformin accounts for one thousandth of the formulated amount. The dry powders are directly mixed with the impeller and the chopper speeds are adjusted based on the mixing state of the materials. The pre-mixing duration is set to 5+5min, with samples taken at intervals for inspection. After the mixing, samples are collected at ten different positions respectively (Figure 5). According to the test results of the samples, the five-minute mixing uniformity RSD is 2.30% and the ten-minute mixing uniformity RSD is 1.28%.
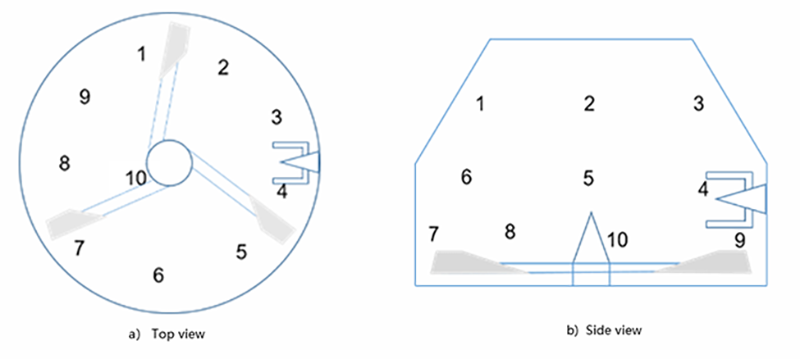
Figure 5: Sampling points
Results
When the API only accounts for one thousandth of the formulation, the simulation of low dose drug mixing uniformity indicates that even a shorter mixing period with the wet granulator ensures a content uniformity RSD well below 3%, outperforming optimal mixing data from other suppliers (Figure 6).
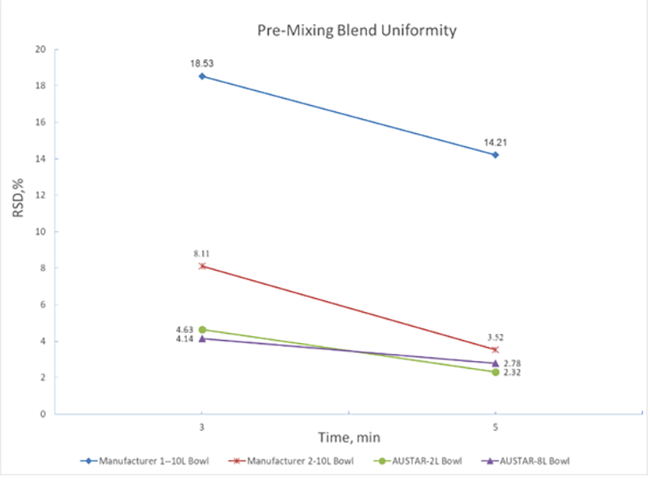
Figure 6 Mixing data of same materials from different suppliers
Summary
The effectiveness of wet granulators in mixing, under consistent material conditions, is heavily influenced by mechanical design elements such as bowl shape, mixing impeller type, and chopper configuration. These factors contribute to performance variations among granulators from different manufacturers. Additionally, understanding and optimizing process parameters under various load conditions are critical.
While extending mixing duration can enhance API uniformity in low dose drugs, excessive mixing should be avoided. Traditional sampling based on time intervals is labor-intensive and time-consuming. Real-time monitoring using Process Analytical Technology (PAT), such as Near-Infrared (NIR) technology, enables rapid determination of optimal mixing times.
Moreover, due to the high potency of low dose drugs, strict containment measures are essential during both research and production phases to prevent airborne emissions of active ingredients and ensure operator safety.
References
[1] CDE: Quality control points of low dose solid drug products
[2] Formulation and Analytical Development for Low-Dose Oral Drug Products
[3] Handbook of Pharmaceutical Wet Granulation_ Theory and Practice (2018)

