"Sticky Web" is a new technique for precision powder dispensing. Howard Biddle, managing director of 42 Technology, looks at its discovery, potential applications and progress towards commercialisation.
Powder handling is technically challenging at the best of times but accurately filling capsules with milligram quantities of potent APIs at the manufacturing speeds and outputs required for a blockbuster product adds another layer of complexity.
Manufacturers have tended to overcome this hurdle by bulking out actives with excipients and using mixtures for tablet pressing or capsule filling but there is considerable commercial interest in developing high speed manufacturing technologies to dispense pure active powders.
"Sticky Web" is a novel precision powder dispensing technology capable of accurately "printing" 0.1 to 100mg of powdered active pharmaceutical ingredients (APIs) with a variety of particle size distributions, onto adhesive webs or surfaces. Accuracies are typically better than ±4% and the approach can deliver commercial manufacturing speeds of up to 60,000 doses/hr.
The discovery came about when GSK first appointed product design and development consultancy 42 Technology (42T) to carry out an independent strategic technology review and to evaluate what credible powder dispensing approaches already existed that could potentially be scaled for high-speed manufacturing. GSK already knew of some commercial systems claiming speeds up to 15,000 doses/hr but they typically involve checkweighing or volumetric techniques and are often unsuitable for some pure APIs requiring careful handling.
The novel technology, now known as "Sticky Web" was first discovered by 42 Technology (42T), then jointly developed in a programme with GlaxoSmithKline (GSK) to target pharmaceutical applications. However, the approach has been shown to be generic and scalable for virtually any market or application requiring high volume, precision powder dispensing, such as: diagnostics, flavours, fragrances, catalysts and other speciality chemicals.
Initially, 42T secured the technology rights for non-pharmaceutical markets but the scope has recently been extended with GSK transferring the worldwide pharmaceutical rights to the consultancy as well.
Sticky Web takes its name from a simple but powerful discovery that when a piece of adhesive parcel tape is dipped into powder and the excess shaken off, the quantity left adhering is directly proportional to the surface area of the tape. The exact quantity depends on the combined properties of the specific adhesive and powder, but even the very first tests showed consistent coverage rates of approximately 1.5mg/cm2 with accuracies better than ±10%.
Those tests were so compelling that GSK and 42T quickly established a joint development team to create a stronger technology platform and improve the dosing accuracy, in some cases to within ±2.5%. The work progressed quickly to develop the approach, several adhesives and designs for a production machine, as well as to secure a number of key patent applications.
Figure 2 (above) shows a simplified schematic of the powder dispensing operation at the heart of the production machine. The web, which is pre-printed with adhesive further up the production line, passes around a drum where powder from a vibrating hopper is applied through drum apertures or masks to these sticky areas. A vibrating paddle taps off any excess, which then drops back into the hopper. The technique works well in accurately delivering uniform coverage for powders that are either free running or have dry clumping behaviours and it is gentle enough for sensitive materials. Some studies have also been undertaken using micronised substrates as a route to lower densities, which would be invaluable for dosing smaller quantities of more potent materials.
Figure 1 (main picutre, top) shows a single strip of coated film web ready for die cutting into individual doses. Each 2cm diameter area shown here contains approximately 4.7mg of API. The substrate is typically around 30µm thick, giving a total coated thickness of approximately 100µm and allowing the powdered films to be easily rolled or folded for encapsulation or further processing as required. The singulation and packaging processes can be fully automated as part of a complete continuous commercial production line.
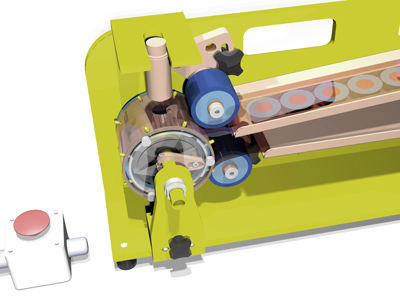
Fig. 3: CAD model of a single lane test rig that can deliver one dose per second. Units can be combined in a multi-lane machine to deliver commercial-scale output
A 3D CAD model of a test rig unit delivering one dose per second is shown in Figure 3 (above). However, further optimisation to improve this rate and a multiple lane production machine with, for example, 16 lines in parallel would deliver around 60,000 doses/hr.
films, adhesives and dissolution
Aside from the powder-dispensing module, the two other main system components are the web or film carrier and the printable adhesive. Edible or inert films have been widely used within the food processing industry for years; they can be safely ingested while being robust and flexible enough for standard web handling. In addition, there is growing interest in thin-film drug delivery and controlled-release encapsulation technologies, both of which are generating unusual new films and materials for potential exploitation within the process.
As part of the development programme, 42T also investigated existing edible pressure-sensitive adhesives (PSA) and developed a range of new substances. These novel adhesives can be printed onto webs, offer instant tack in the dry state and gently adhere to the steel powder dispensing drum but then peel without leaving any residue. In addition, they offer good powder adhesion and dissolve readily in water.
Figure 4 (below) shows a 20-times magnification of a typical powdered area generated with one of these new adhesives: note the clean edges, even powder distribution and adherence of large and small particles within the sample.
One of the most interesting features of this novel dispensing approach is the potential for improved release of active powders with a tendency to agglomerate during dissolution when they are delivered via powder-coated films versus capsules. Some initial development work has suggested a marked increase in bioavailability in the bulk system but further validation studies would be required for specific adhesive and powder combinations.
future developments
The focus of the development activity to date has looked at using edible flat carriers, likely to be the preferred route for pharmaceutical applications, but the adhesive could just as easily be printed onto surfaces, bubbles or tablets that are subsequently precision-dosed with powders. Two or more substances could even be combined into the same delivery package.
The approach is also available for virtually any industry requiring accurate, high volume dosing of powders to improve existing manufacturing processes or generate innovative new products or processes.
Sticky Web offers considerable promise for high speed production environments where online inspection systems could be used to validate every powdered area and to improve process feedback, increase efficiency and reduce costs.
GSK was initially pursuing pharmaceutical applications as part of its commercial partnership with 42T, with the consultancy looking at all other markets. However, in line with GSK's strategy, the company decided to extend the scope of the licence. Over the coming months it will support 42T in helping to identify and secure additional potential development partners to realise the full commercial value of this novel technology.
And for a technology that started life with a eureka moment involving sticky tape and powder, the commercial possibilities for a number of major markets are virtually unlimited and waiting to be discovered.
Howard Biddle is md of 42 Technology - a product design and development consultancy, based in St Ives near Cambridge, and specialising in creating innovative products, processes and instrumentation
