As drug development is a lengthy, complex and costly process, entangled with a high degree of uncertainty whether a drug will actually succeed, an enhanced ability to handle ever-growing pipelines and identify active compounds that need further investigation at an early stage of candidate selection is vital.
The future of medicine
Although companies have steadily increased their expenditures on research, the number of new drug approvals has been modest for many years. Whereas cost is usually cited as the main explanation, other factors must also be considered, such as increasing regulations, generic competition and reimbursement challenges.
Nonetheless, biopharmaceuticals have been identified as a significant opportunity … and participating in the emerging markets for these therapies is unanimously considered to be contributing to the future of medicine. In the face of current industry hurdles and drivers, the global biopharmaceuticals market is growing considerably and estimated to reach a value of $400 billion by 2025, including biosimilars.
In particular, with regard to chronic and/or severe clinical conditions such as multiple sclerosis, rheumatoid arthritis or cancer, biopharmaceuticals are regarded to be next-generation treatments.
Increasing numbers of protein drug candidates
A number of patents for well-established blockbuster biologics will expire in the foreseeable future, clearing the ground for the development of a growing number of biosimilars. Hence, alternative products for existing blockbuster originators will be great opportunities for developers.
At the same time, the importance of new biological products as therapeutic options for various diseases is expanding exponentially, and the development of first-in-class new biological entities (NBEs) is an economically appealing challenge.
Without a doubt, there is a distinct need for more specific biopharmaceutical therapeutics in the market, especially in nascent personalised medicine, whereby patients with serious rare diseases can benefit from the tailored biologics in the future.
Paradigm shift in analytics
Instead of pinning their hopes on one singular product, developers are increasingly putting more and more effort into the evaluation and identification of a vast scale of candidates, aiming to develop a larger number of products with smaller expected economic results on an individual level … but with promising sales as a whole.
Increasing pipelines mean greater demands for analytics. The more candidates you focus on during the selection process, the less the application of traditional analytics will deliver the required results. Only the most detailed knowledge at the earliest possible stage of development will guarantee the best and most reliable choices with regard to the developability of a candidate.
This includes, for example, the earliest possible detection of critical quality attributes (CQAs) and the comprehensive understanding of their specific linkage to a molecule’s mode of action (MOA).
Limiting factor: clone selection
We believe that the limiting factor in terms of introducing new biotechnology derived products is no longer the search for new drug candidates … but the selection of those with the highest potential for commercialisation. Therefore, efficient and rapid developmental strategies need to be devised that cover all aspects of drug development (such as fermentation, purification, characterisation and formulation).
The multi-attribute method (MAM) is a powerful approach that helps to meet this challenge, allowing the extraction of a large number of different protein parameters a within a single analytical approach (Figure1).
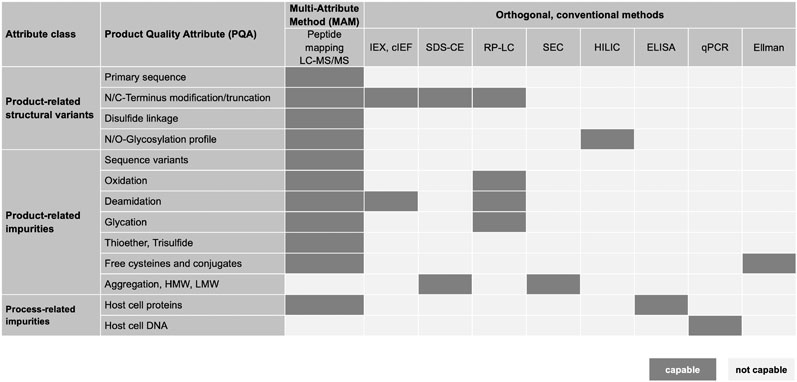
Figure 1: The multi-attribute method’s ability to analyse many product quality attributes (PQAs) in parallel could replace conventional approaches
Process observation, data gathering, differentiation and interpretation are basic steps in product development. As the complexity of biotherapeutics continues to increase, the standards for analytical assays designed to provide the required data and high-quality insights are increasing as well.
MAM has the potential to replace conventional approaches and reduce the development time span while simultaneously increasing the quality of the information obtained. Peptide mapping with quantitative LC-ESI-MS provides specific information on quality attributes, such as truncation, deamidation, oxidation and glycosylation.
Accelerating identification
Time savings, cost reduction and risk minimisation are the undisputable triumvirate of success in the development of biopharmaceuticals. At the same time, developers have more simultaneously ongoing projects than ever — and their number will continue to rise.
With regard to rising cost pressures and increasing demands for all-enclosing analytics, a potent partnership ranging from cell banking and clone selection to comprehensive process development and release testing services can help to increase both capacity and specificity.
High-tech approaches, in particular MS technologies such as MAM, are able to deliver very concise and specific information from late research/early development onwards while, at the same time, allowing for high throughput.
MAM: deeper understanding and detailed knowledge
Low-resolution MS techniques have less specificity and are unable to determine certain critical modifications, especially when there are no chromatographic differences in modified and unmodified peptides.
Digesting proteins into peptides, which are then monitored meticulously, the MAM workflow (Figure 2), based on high resolution and accurate mass techniques, not only provides a multitude of data in a comparatively short time, it also provides a deeper understanding of the manufacturing process, potential unwanted modifications and their possible impact on the product.
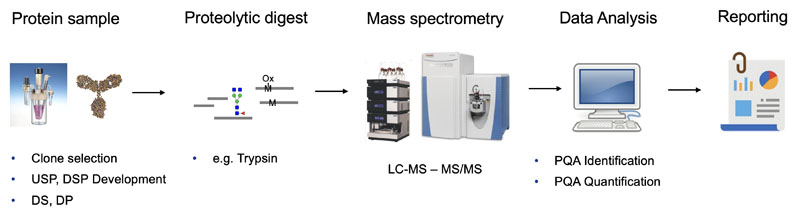
Figure 2: MAM workflow
Classical HPLC methods are able to show differences in sample profiles but tend to associate the detailed information with where they’re derived from. The multi-attribute method enables highly accurate relative quantification of molecular details through peptide mapping (with sequencing of the peptides by MS/MS fragmentation).
The MAM workflow permits a detailed search for components referring to a large variety of critical quality attributes. The information gathered not only gives information on product quality attribute (PQA) modifications — such as post-translational modifications or attached N-glycan species — that occur owing to specific intentional changes within the process development, but also provides evidence about the exact location of the PQAs in the protein sequence.
The influence of the manufacturing process on the biopharmaceutical can therefore be revealed to a very detailed degree.
Partnerships increase efficiency
Exceedingly high development costs emphasise the need to ensure that existing methods are as efficient as they can be. Only by using a comprehensive approach are rapid evaluations of data and well-structured summaries of observations and results feasible.
The challenge to meet these high standards and the increasing complexities of markets, long-term business strategies and release requirements are placing a stronger emphasis on the expertise and experience of an external partner. Outsourcing support can not only lower the costs of research, development and manufacturing, it also enlarges the scale of testing methods by getting a well-equipped and experienced partner on board.
Based on extant and currently evolving technologies, contract research organisations (CROs) such as Protagen Protein Services (PPS) provide support across a broad range of specific activities, including characterisation, stability and comparability testing, as well as bioanalytical and release testing.
A strategic partnership with a single CRO, typically lasting several years, helps reduce expensive tech transfer, saves internal resources and ensures access to state-of-the-art equipment and a wealth of experience. Paving the way for increased efficiency and tailored development and testing processes, long-term partnerships might be the key to success.
