Maintaining sterility during the production of injectables is a major challenge with conventional technology. Regulatory actions against substandard injectable drug manufacturing sites have resulted in shortages of drugs used to treat potentially life-threatening conditions.
With demand for sterile injectables ever increasing, there is tremendous pressure on pharmaceutical manufacturers to increase productivity while simultaneously maintaining strict safety and quality standards.
Recent advances in robotics enable the transition from human-centred production to closed and entirely hands-off automated systems throughout many manufacturing industries. This is an important development for the pharmaceutical industry wherein the human operator is the greatest source of contamination in aseptic processing.
In this article, Ross Gold, Director of Aseptic Filling, examines how advances in aseptic design are eliminating contamination risk and how fully automated and completely closed aseptic filling systems provide significant and quantifiable improvements to sterility assurance for injectable pharmaceuticals.
Eliminating risk through design
Regulatory bodies recognise that automated isolator technology enables superior aseptic processing and patient safety. However, conventional aseptic filling technology relies on human operators that manipulate critical aseptic processes through glove ports.
In addition, conventional aseptic filling lines have a large footprint with a complex set-up that necessitates extensive environmental monitoring for contamination control. For instance, particle-generating elements require multiple air sampling sites to ensure that product quality is not compromised.
Advances in automation technology — coupled with clever engineering solutions — have driven the development of standardised, completely closed robotic isolators.
In contrast to traditional aseptic filling lines, a “closed workcell” offers an isolated environment with an exceedingly low risk of contamination — as well as increased containment of the drug product.
The identification of key hazard pathways in conventional aseptic filling lines has guided the design of these closed workcells. Eliminating the need for operator intervention in automated aseptic filling means that glove ports can be designed out. This technology is referred to as a “gloveless” system. Instead of direct human intervention, process controls are managed via an intuitive touchscreen.
That said, a common concern that remains with automating pharmaceutical production is the generation of non-viable particulates by robotics, as well as the fact that they can be difficult to sterilise. This concern can be resolved by taking inspiration from the semiconductor industry, in which the use of closed robotic workcells is routine.
Selecting advanced and high-quality robotics, minimising the number of moving parts and positioning them intelligently within a closed workcell can create a system that will meet particle generation regulations and is easily sterilised by automated vapour phase hydrogen peroxide protocols. Although the design features of gloveless isolators clearly operate in theory, do the intended benefits translate to the real world?
Welcome to reality: reducing contamination
Answering this question represents a common goal throughout the pharmaceutical industry and, especially, for the Aseptic Filling Workcell User Group (AFWUG), which represents users of standardised, closed robotic workcell technology for aseptic processing.With more than 40 participating companies that regularly collaborate on the implementation, environmental monitoring and regulatory aspects of aseptic technology, the group recently turned its efforts towards exploring the real-world benefits of gloveless isolators.
Eight independent companies from the AFWUG that use the SA25 Aseptic Filling Workcell from Cytiva aggregated their production data for analysis. In a recent talk at ISPE Aseptic 2022 (North Bethesda, MD, US), two members of the user group delivered their findings regarding the risk of contamination, non-viable particle counts and the ability to handle relevant container formats.
Joseph McCall of ADMA Biologics and Kevin Gadient of Emergent BioSolutions presented an analysis of microbial air samples from 238 batches and post-fill needle swab data from a subset of 185 batches.
All samples showed no contamination, illustrating the highly aseptic environment of a gloveless closed workcell. In the SA25, this derives from sealing the needle/swab assembly after filling to mitigate the need for further interaction. ISO 5 regulations are an ultra-clean classification reserved for only a few industries and provide guidance on non-viable particle counts at 5 µM and 0.5 µM.
In the workcell study, non-viable particle counts for 256 batches indicated that there was a >99.99% probability that particle sizes will fall below the ISO 5 limits for both 5 µm and 0.5 µm (Figure 1).
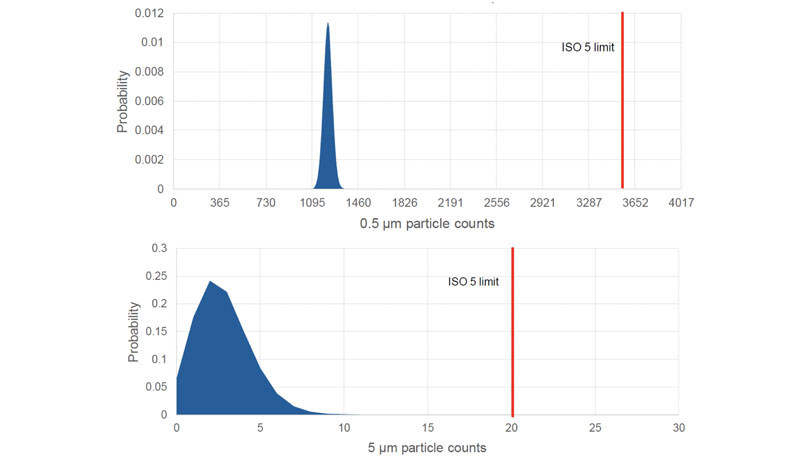
Figure 1: Poisson distribution plot for the number of (A) 0.5 µm and (B) 5 µm non-viable particles, indicating that the probability of out-of-specification events occurring is <0.01%
A further challenge when designing an aseptic filling station that’s suitable for broad application in the pharmaceutical industry is ensuring that it can manage the huge variety of container formats used. Joseph and Kevin highlighted that an automated closed workcell could cover all 178,099 units across multiple formats (ranging from vials to syringes and cartridges) in the study (Figure 2).
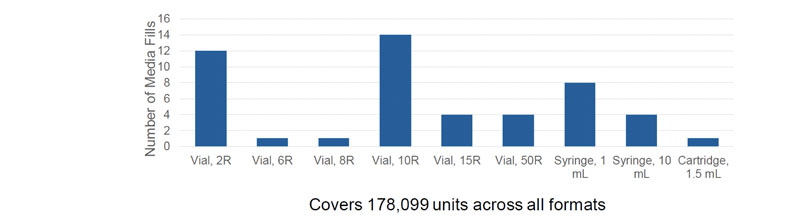
Figure 2: Container formats qualified via media fills using the SA25 Aseptic Filling Workcell from Cytiva
Collectively, the aggregated data from the AFWUG collaboration suggest that a closed robotic filling process within a gloveless isolator provides significant benefits to sterility assurance.
The future of aseptic filling is robotic
Ensuring the sterility of injectable drugs is critical because of their use in treating at-risk (immunocompromised) patients. As the injectables market continues to grow, manufacturers are under increasing pressure to increase capacity without compromising quality and safety.
The automation of critical processes offers the pharmaceutical industry a means to reduce risk with a range of therapeutic modalities — from monoclonal antibodies and mRNA vaccines to gene therapies and therapeutic peptides.
Simultaneously, systems such as the SA25 can help to boost production while maintaining safety and quality standards. The ability to provide safe, sterile drugs that meet demand will drive healthcare forward and benefit patients worldwide.
