Dr Sarah Houlton reviews recent development of gene manipulation technology and its potential application in providing new medicines
Unravelling the sequence of the human genome has posed as many questions as it has answered for drug discovery scientists. If they can work out how the genetic code translates into biological functions, it will aid the identification of potential targets for drug intervention. Techniques that allow the genome sequence to be accurately modified are extremely important tools in this work – if genes can be ‘knocked out’ precisely and quickly, it will greatly facilitate the search for their function, and help pinpoint the biological effect of stopping the gene’s activity.
Gene manipulation used to rely on random, non-targeted methods, such as chemical-induced mutagenesis and ionising radiation. While these altered the genome, the ability to predict and direct the mutations was limited, and there was a degree of luck involved in creating an interesting mutation. Over the years, more targeted methods have been developed, notably those that use homologous recombination. This is a naturally occurring mechanism for repairing DNA that takes place in most cells, and uses a duplicate chromosome as a template for repairing a damaged gene.
The power of this technique became clear when it was applied to mouse embryonic stem cells. By introducing foreign DNA as a repair template, researchers found it was possible to make accurate changes to the gene sequence, by changing, deleting or adding genes. This work led to the 1989 creation of the first ‘knockout mouse’ work, which led to the 2007 medicine Nobel prize for Mario Capecchi, Martin Evans and Oliver Smithies. Because one or more genes are absent from their genome, these mice have proved to be important research tools for investigating the function of genes; changes in the mouse’s condition, caused by that gene deletion, can be studied.
The early 1990s saw the development of a new, highly selective technique for manipulating genes. In higher-level animals, the technique is a thousand times more efficient than the cells’ own homologous recombination process. It was based on work on zinc finger protein domains. A zinc finger is a small protein, containing up to about 30 amino acid residues, that co-ordinates at least one zinc ion. This zinc ion templates the protein, stabilising its folded structure. These naturally occurring complexes each bind to a specific set of three nucleotide bases.
If several of these zinc fingers are connected together, and then fused to an endonuclease FokI protein that cuts DNA, a very directed cut can be made in the DNA. For example, if four zinc fingers are joined together, it will then bind a very specific 12 base pair sequence within the DNA, and then cut the DNA at the end of this sequence. Sheer force of statistics means it is extremely unlikely to bind and cut anywhere in the DNA other than the desired location, because it is unlikely that the same long sequence of bases will occur elsewhere in the gene. These zinc finger nucleases (ZFNs) are designed in pairs that bind to adjacent sequences in the target DNA in order to cut both strands, and can be considered as targeted genomic ‘scissors’.
Cutting the DNA is only half of the story. The double strand break in the DNA caused by the ZFN can be repaired in one of two ways – non-homologous end joining (NHEJ) or homology-dependent repair (HDR). NHEJ occurs if no DNA repair template is available, and up to a fifth of cells whose DNA has been cut in this way will be mis-repaired with a gene deletion. If a repair template is introduced along with the ZFN, then the new DNA will be introduced in up to a fifth of cells, thus giving gene integration at the target site.
Gene knockout – or deletion – is an extremely powerful tool for discovering the functions of specific genes, and ZFN enables this to be done very accurately. By creating cells, or even whole organisms such as knockout mice, where a gene is missing, it is possible to probe what the gene does. For example, this technology has been applied to zebra fish, by designing ZFNs that target the golden and no tail/Brachyury, or ntl, genes.1 About a third of embryos that had been injected with ZFNs targeting the golden gene lost this gene, leading to unpigmented cells in the eye. Similarly, 27% of those where the ntl gene had been knocked out had stunted tail growth.
The process is similar for ‘knocking in’ (adding) a gene, with a custom ZFN to cut the DNA being delivered along with a homologous donor. This donor is a plasmid – a non-chromosomal DNA molecule – that is homologous with one side of the cut DNA at one end, and with the other side at the other end. These arms of homology need to be only about 750 to 800 base pairs long, which is very much shorter than the homology required for alternative methods of knocking in genes; for example, with mouse embryonic stem cells, homology in the order of 10,000 base pairs is needed. The extra gene that sits between the homologous arms will then be integrated into the repaired DNA, giving a gene addition.
A third possible use of ZFNs is in carrying out single base pair modifications. Targeted gene correction has the potential to become a very powerful technique for researching the impact of gene mutation on an organism. It could also be used to improve cell lines that are used in research, or even correct genetic defects that lead to disease in vivo.
promising in vitro results
Gene therapy has already been investigated in the treatment of X-linked severe combined immunodeficiency disease (SCID), or bubble-boy disease, as it is caused by mutations in a specific gene, IL2R. This disease has also been used as an in vitro model system to look at the potential of using ZFNs to correct genes, and results were promising – targeted alterations were made to the gene in more than 18% of recipient cells.2 In about 7% of the cells, the genetic modification was seen on both X chromosomes.
However, if gene therapy is going to be able to alter a person’s genetic blueprint, then progenitor and stem cells have to be modified ex vivo by gene transfer, and then cultured and re-infused into the patient to give a stable, heritable genetic change.
This can be achieved using a viral vector, and the potential of ZFNs here has been shown in another study in SCID, where genes are delivered using integrase-defective lentiviral vectors.3 Permanent, heritable modifications to the IL2R gene were made both in haematopoietic progenitor stem cells and human embryonic stem cells, showing the technique’s potential.
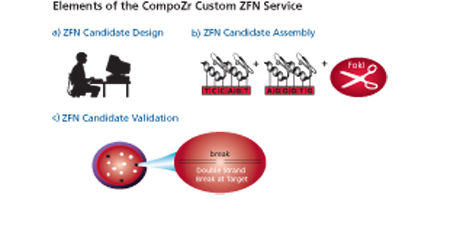
Figure 2: CompoZr ZFNs make the modification of cell line a straightforward lab process
CompoZr ZFNs, from Sigma Life Sciences, were designed such that creating modified cell lines using ZFNs is a straightforward lab process. The technology was licensed from Sangamo BioSciences, which is using ZFNs itself to develop gene regulation and correction therapeutics.
To use CompoZr ZFNs, the ZFN pair is first delivered into cells, using standard transfection techniques such as lipid-based transfection, electroporation and nucleofection. Within a few days, ZFN mediated editing will have taken place, and the cells can be assayed to determine the rate of mutation. Ultimately, between 1 and 20% of the clones will have the mutation, and in four to seven weeks the individual clones will be ready for screening.
knockout rats make better models
While knockout mice have become commonplace tools for pharmaceutical researchers, it is still early days for knockout rats. Rats are, on the whole, better models than mice for many human diseases, but because rats are more complex organisms, the methods used to make knockout mice proved too inefficient to make rats with specific gene deletions.
The introduction of ZFN technology has changed all that, making it possible to create knockout rats. The same technique has also made the process of making knockout mice more rapid.
Last July, a team led by Howard Jacob at the Medical College of Wisconsin announced that the first targeted gene knockout rat had been created, using ZFN technology. Working with scientists at Sage (Sigma Advanced Genetic Engineering) Labs, Open Monoclonal Technology and Sangamo, the group was able to knock out an inserted reporter gene and two native rat genes without any noticeable effects on other genes, and these changes were passed on to the rats’ offspring.4
It is possible to breed knockout rats (and mice) in about four months; this is much faster than embryonic stem cell technologies – it took about a year to create a line of knockout mice using the latter methods.
The technology is already being put to good use. Sage has received funding from the Michael J Fox Foundation to create knockout rats for use as discovery and preclinical research models. Mutations in five separate genes are strongly connected to Parkinson’s, and five different knockout rats, each with one of these genes deleted, are being developed. Scientists hope that these new models will give them a better understanding of the disease at a molecular level, as well as provide physiological insights.
potential in biologics manufacture
Cell culture is the mainstay of monoclonal antibody and recombinant protein production and is becoming increasingly important in the manufacture of vaccines. Biologics manufacture is on the rise and biologics include three of the current top 15 sellers – Enbrel (etanercept, Pfizer), Mabthera (rituximab, Roche) and Humira (adalimumab, Abbott).5
On the vaccine side, there has been a push, through both government funding and industry initiatives, to move away from using eggs in vaccine manufacture and use cell culture instead. This would increase capacity – the recent scramble to make H1N1 influenza vaccine being a case in point – and also reduce the potential for cross-contamination from the eggs themselves.
ZFNs have potential to improve all biologics manufacture. For example, they can be used to create modified cell lines that are more productive, enabling more antibody or vaccine to be produced in a smaller reactor, thus saving both time and cost. One way in which productivity could be increased is by knocking out genes that induce apoptosis. This would give the cells a longer lifespan, and thus increase the yield.
They could also be used to modify the cells to make them more reliable with consistent productivity, for example by including a post-translational modification of the protein such as glycosylation – something that would please the regulators as well as the manufacturers.
Modified cell lines have great potential in the hunt for new medicines. They provide the ability to explore whether a gene has potential as a therapeutic target, and to create in vitro screens to use in a search for a hit compound. The speed and accuracy of using ZFNs to create these cell lines can reduce the time it takes to find that all-important initial hit.
references
1. Y. Doyon et al. Nature Biotechnology, 2008, 26, 702
2. F.D. Urnov et al. Nature, 2005, 435, 646
3. A. Lombardo et al. Nature Biotechnology 2007, 25, 1298
4. A.M Geurts et al. Science 2009, 325, 433
5. Data from IMS Health, 2008 global sales
