Recent heightened interest in MR formulations throughout the pharmaceutical industry necessitates a more profound understanding of how optimal drug release can be achieved through polymeric excipients, notably hydroxypropyl methylcellulose (hypromellose or HPMC).
In this article, True Rogers, Fellow, Pharma Solutions and Matthias Knarr, Research Scientist, Pharma Solutions at IFF, highlight key considerations when selecting HPMC for MR hydrophilic matrix tablets (Figure 1) and provide insights for formulators seeking to tailor release profiles to meet specific performance requirements.
Understanding HPMC chemistry
The development of a consistent and robust hydrogel layer is critical for MR performance from a hydrophilic matrix. When the tablet contacts aqueous media, the HPMC particles near the surface hydrate, swell and coalesce to create a swollen hydrogel layer around the matrix core.
At this initial stage, the active pharmaceutical ingredient (API) close to the tablet’s surface is released during hydrogel formation. However, most of the API dose is liberated during an extended period from, or through, the swollen hydrogel layer via combined diffusion and erosion mechanisms.
For this reason, the ability of HPMC to form a swollen hydrogel layer around the matrix core is fundamental to achieving optimal MR performance.
There are multiple HPMC chemistries to choose from, including HPMC 2208, 2906 and 2910. Each one offers poor solubility to insolubility at temperatures greater than 70 °C and solubility at temperatures lower than 60 °C.
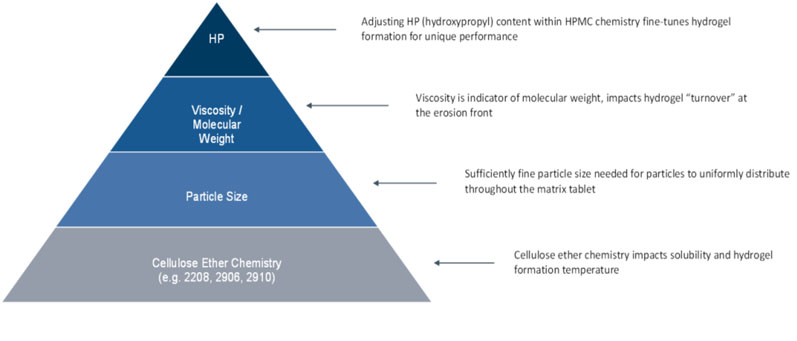
Figure 1: HPMC attributes impacting MR performance
Furthermore, HPMC chemistry impacts the temperature-dependent hydration of HPMC and, therefore, hydrogel formation at physiological temperature (37 °C).
HPMC 2208 has emerged as the polymer of choice for MR matrices owing to its ability to readily hydrate at 37 °C. HPMC 2208 dissolves at 10–15 °C above physiological temperature, so it readily hydrates and swells at body temperature.
Other HPMC chemistries dissolve at temperatures closer to 37 °C, making them less amenable to rapid hydrogel formation at body temperature.
Ensuring robust performance: the role of particle size
Another key aspect of contiguous and robust hydrogel formation around the matrix tablet — and therefore robust MR performance — lies in attaining a percolating network of HPMC throughout the matrix tablet.
This is a continuous lattice of uniformly distributed HPMC particles in close enough proximity to hydrate, coalesce and form the hydrogel layer.
HPMC particle size affects the threshold at which a uniform lattice throughout the matrix is attained; achieving the percolation threshold is critical for consistent hydrogel layer formation and “turnover” during MR.
Compared with a fine particle size grade of HPMC, a coarse particle size would necessitate higher HPMC concentrations to attain the percolating lattice throughout the matrix tablet.
Impact of molecular weight on MR duration
MR duration is another important consideration and can vary from 6–24 h, for example, depending on the API, its physicochemical and PK properties, and the molecular weight (or viscosity grade) of the HPMC.
HPMC viscosity grade selection plays a pivotal role in release duration because it influences hydrogel turnover at the erosion front of the swollen matrix layer. The higher the HPMC molecular weight, the slower the hydrogel turnover at the erosion front, resulting in a longer release duration.
Typically, poorly soluble APIs, such as acetaminophen, depend on erosion-dominated release through the swollen hydrogel layer (with lower viscosity grades). More soluble APIs, such as metformin, rely on diffusion-dominated release (with higher viscosity grades).
Fine-tuning release profiles: why hydroxypropyl (HP) content matters
In some instances, particularly when unique release kinetics are necessary for certain APIs, such as gliclazide, further customisation may be needed to achieve targeted MR performance. In these cases, tailoring for unique performance can be accomplished by adjusting HP content within a particular HPMC chemistry.
This adjustment further modulates the hydrophilicity of the HPMC polymer, which can then be utilised to fine-tune the hydrogel formation and release kinetics.
In these cases, an increase in HP content has correlated with a rise in HPMC dissolution temperature and, therefore, caused even more rapid hydrogel formation at body temperature.
This subsequently results in zero-order release kinetics being attained in less than 8 h for a unique API — such as the previously mentioned gliclazide. Achieving cutting-edge, high performance MR tablets that prioritise patient needs doesn’t need to be complex.
But it does require a specialist in excipients. As a trusted partner in this field, IFF harnesses its polymer expertise and problem-solver mindset to support pharmaceutical manufacturers throughout the entire drug development pipeline.
