Biologics producer MedImmune won a Facility of the Year Award for Project Execution in 2011 for the build of a large-scale mammalian cell culture-based production facility in Maryland, US. This article highlights its winning concepts.
In the world of biologics, MedImmune has one of the most robust pipelines. The company currently has approximately 100 biologics in r&d, including 16 in clinical development and 11 in preclinical development. The challenge of having such a robust product pipeline is the operational capability and flexibility required to manufacture such a diverse group of products with a wide range of titres.
To enable production of forthcoming products, the company decided to build and license a flexible, large-scale mammalian cell culture-based production facility adjacent to its existing Frederick Manufacturing Center (FMC), Building 636. This would allow it to leverage the expertise and systems already in place at FMC, which had been used to produce successfully its blockbuster drug Synagis (palivizumab) for the past 12 years.
The new facility, Building 633, is designed to house 337,000ft2 of administrative, production, warehouse, lab and utility space. To accommodate future growth, internal expansion capabilities of 100,000ft2 of production space have also been included.
The company wanted a flexible facility that could accommodate a wide product titre range of 0.5 to 7.0g/litre. Though a 10x process range has been achieved in practice before in the biopharmaceutical industry, MedImmune believes that Building 633 is the first large-scale facility in the industry able to produce a 14x process range up to 7.0g/litre.
The company planned for a modular approach to integration and engaged all equipment skid manufacturers early in the Process Control System (PCS) development process. It distributed the S88 model to the skid producers to ensure development of common equipment and control modules. To verify that these skids would flawlessly integrate into its PCS infrastructure, it developed a FAT PAC – a portable package of servers that replicated the high-level process network, enabling the company to test the equipment at vendor sites.
A complete, isolated replica of the PCS was developed to allow validation activities to be performed at the same time as equipment validation and shakedown, to reduce risk. Replication of the manufacturing equipment operation using PLC controllers in a virtual environment proved to be an efficient and effective method of PCS validation.
The company also planned for successive shakedown (test) runs before the start of qualification. Shakedown activities, which commenced during commissioning and qualification, took precedence in the project schedule. This approach maximised operator on-the-job training and provided opportunities to identify issues.
The primary design objectives for Building 633 were the creation of process flexibility; the mammalian cell culture-based monoclonal antibody facility had to be able to support manufacture of multiple products.
The company used a closed system approach, where feasible, to allow simultaneous production of multiple batches or multiple similar products within process areas. This design would allow for concurrent manufacturing in cell culture and campaigned manufacturing in purification. The design also allowed for staged product changeover to maximise equipment uptime. Manufacturing capacity is based on a production module of four 15,000L production bioreactors with a 12,500L working volume, and the supporting purification capacity to provide bulk substance output.
Three independent inoculum expansion labs are provided to allow for concurrent expansion of up to three different cell lines. Each inoculum lab is self-contained, with single-pass airflow to eliminate cross contamination.
The seed bioreactors are arrayed in redundant trains, with the flexibility to use any given seed bioreactor in seed train development. Either final seed bioreactor may be used to inoculate any of the four production bioreactors. The seed train allows operation at full capacity, even at minimum design cycle times, while allowing for backup seeds at reduced capacity.
The initial seed bioreactors accommodate a feed up strategy, thus requiring an operating range of 30–100%. Process throughput design is based on a bioreactor cycle time range of 11–20 days. All bioreactors are capable of operating from 40–100% of working volume by utilising dual impeller design.
The redundant design of the bioreactor hall requires flexibility in transfer paths to accommodate a wide range of processing scenarios and redundancy in the installed base of harvest and downstream process equipment. The downstream process areas were designed to meet cell culture output, such that production bioreactors should be rate-limiting to maximise equipment utilisation.
The purification process areas are designed to support the wide range of production scenarios. As installed, the purification equipment includes the process capability for three chromatography steps and a variety of viral inactivation, viral filtration, and ultrafiltration processes.
To support quick turn-around time in purification, two sets of chromatography skids, chromatography columns, and purification vessels were installed. Chromatography steps were to be limited to five cycles, with the following additional requirements:
- Chromatography column sizes provide range from 100–180cm diameter with provisions to use 200cm columns, if required.
- Installed SS pack-in-place columns are rated for 5 bar.
- The facility provides the ability to install and remove columns with a clear path from purification process areas to the loading dock.
Chromatography transfer panels were built with the flexibility to configure chromatography control skids with any of eight buffer inlets, two product inlets as well as WFI, 0.1N, and 1.0N sodium hydroxide. In addition, multi-port valves were installed on the chromatography control skids to allow time-saving stacking of buffer and product line CIP and SIP through transfer panels and chromatography skids.
All ultrafiltration equipment was sized to accommodate the wide range of possible final bulk substance volumes.
process models
Three model processes were chosen as a design basis, including that for the company’s existing product, Synagis, and two pipeline products that were chosen because of their extended range of process requirements. All three processes were modelled using Excel-based process flow diagrams with mass balances. The spreadsheets were set up to allow simple and rapid ‘what if’ analysis of process variables.
This analysis yielded not only a preliminary equipment list and sizing basis, but also forced certain process decisions. For example, the microfiltration process currently used in creating Synagis was neither practical at a large scale, nor suitable for future products.
MedImmune chose to design for centrifugation-based harvest but also to allow flexibility for future microfiltration-based processes. A microfiltration (MF) feed tank was pre-installed to address this eventuality. In addition, piping connections were installed to allow for rapid installation and qualification of a future MF skid.
The sizing basis also uncovered large footprint and height requirements for depth filtration following centrifugation. The company decided to utilise Millipore POD depth filters (new technology at the time) to conserve space, reduce CIP requirements, and enhance flexibility. The company provided for a total of four POD skids for two filtration steps and custom designed a piping control module to operate any or all of the skids.
The large titre range also requires a large range of volume capacities in the buffer hold tanks and product hold tanks. The resulting equipment installation includes buffer prep tanks ranging in size from 500–25,000L, buffer hold tanks ranging from 2,200–25,000L working volume, and nine product hold tanks ranging from 2,200–13,600L. To support the variety of tank sizes, a process piping arrangement of transfer panels and multi-port valves was designed to allow selection of any group of source and destination tanks, appropriate for specific product and yield.
Finally, the range of starting titres as well as a range of final bulk substance concentration require sizing flexibility in the ultrafiltration (UF) skid and feed tanks. Rather than dedicated small and large UF skids, the company designed a dual pump/dual cassette UF skid with flexibility to feed from two UF feed tanks of different size.
After completing IOQ, the company performed a process capability study to verify the as-built plant capabilities.
The sheer magnitude of the facility dictated the complexity of the PCS. The team decided that the PCS platform would be based on the Rockwell family of hardware and software and is a fully integrated, custom installation, designed as a GAMP5-Category 4 system. The PCS needed the capabilities for control, monitoring, alarming and data collection of more than 40 production skids; of all process piping and transfer panels; holding tanks, CIP and SIP equipment; and of critical utilities.
plant-wide controls
In addition to an innovative approach for integrating the various skid systems into the PCS, the automation team developed a plant level strategy for security and data collection.
Rockwell’s Factory Talk Historian serves as a common repository for process data and alarms from all systems. A Microsoft SQL Server tracks process events, for example, the start of a cleaning cycle. Microsoft SQL reporting services are used to generate reports by querying both sources of data: the Historian and the SQL database. This architecture provides a platform upon which MedImmune will be able to transition easily to EBRs at a future date without a complete redesign or revalidation of each skid and the PCS.
The architecture also allows all data and alarms to be displayed on any HMI in the facility. Operators need go to only one HMI to view operations in any part of the system.
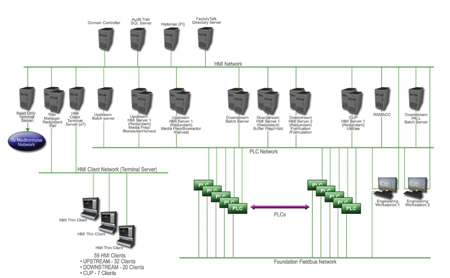
This architecture allows all data and alarms to be displayed on any HMI in the facility. Operators need only go to one HMI to view operations in any part of the system
During automation system startup in a cGMP environment it is typical practice to leverage work completed in FATs, installation, and commissioning. More often than not, these activities are limited to hardware IQ checks. Functional OQ test validity is always in question due to lack of code control.
Installation and testing of a system this size, with several million lines of code and more than 40 different skids, is a daunting task to manage. In addition, the leveraging strategy dictated by MedImmune added a complexity to start-up activities that posed a major challenge to the team: how to track, maintain and control PCS code revisions while simultaneously performing leveraged start-up activities. The automation group took a unique approach in adapting an off-the-shelf application to meet its needs.
The team created a Configuration Management Plan at the onset of planning. This document set the process for managing all application files. A key aspect of the plan was the use of an automated configuration management tool. Rockwell’s Factory Talk AssetCentre is a program that manages all coding such that it requires programmers to ‘check out’ code from a repository and has automatic revision control. This allows an auditor to go back to any point in time and see what changes were made to the code throughout the development and testing process.
AssetCentre was used to control all configuration files, including PLC applications and HMI configurations, for both skids as well as stickbuilt systems beginning immediately after the FAT for each component. The system integrator configured the software based on a configured hierarchy of all PCS components.
The use of an automated configuration management tool quickly proved valuable during commissioning and start-up, because many changes were identified and implemented during this phase. At one point, 15 control engineers were accommodating various change requests to the PCS code.
This process continued through validation, with a parallel test system configured as a mirror image of the production system that was being validated. Both systems used a common AssetCentre server. Following validation of each skid or process area, the associated code was versioned again and an automatic schedule for upload and compare was established.

The company created a complete duplicate of the production system to enable equipment testing and training
This schedule provided for automatic comparison of the actual code running in the production system with the locked, effective, master version. Any deviations between the two versions initiated an automatic email to key team members. In addition to control of PCS code, this automated configuration management system has also been used to control batch file configuration management.
An issue that all facilities start-up teams deal with is the time required to start up and debug applications, train production operators and run test batches. These steps in the process are always competing for time on equipment. They typically overlap each other and the start up of Building 633 was no different. However, with the time constraints dictated by the aggressive schedule, each group needed to run specific tasks 24 hours a day, seven days a week, concurrently.
Using a Rockwell SoftLogics solution the company created a complete, all-inclusive duplicate of the production system. The operational qualification of the S88 system was performed extensively on the simulator. This allowed commissioning and shakedown runs to be carried out on the actual equipment, freeing up valuable time.
Since both the production system and the simulator were being used in parallel, this dramatically reduced the overall time required to execute the project.
The company also planned early to implement a successful Clean Build programme, with increasingly restrictive cleaning requirements as construction progressed into ICQ and start up. Implementing Clean Build early in the process provided a controlled environment in which to construct mechanical, electrical, process piping and cleanroom finishes.
The team recognised that the commencement of test manufacturing runs and PV runs would depend on the complete installation and testing of required process and support equipment systems, integration of CIP, SIP and transfer piping flow paths and all processes. They also recognised that these activities would have to occur under the constraints of the ICQ activities.
shakedown schedule
To mitigate these risks the company needed to ensure sufficient run-time on the equipment prior to the start of PV runs. It decided to lay out a schedule of progressive shakedown phases that used more unit operations during each successive phase. Scheduling backwards from the committed PV completion date, seven shakedown phases were planned to allow operators sufficient run-time on all of the equipment, as well as suitable time for Manufacturing Sciences & Technology (MS&T) staff to characterise all processes to assure easy scale-up.
Although the focus of the shakedown runs was to help scale-up in cell culture, the company also gained a great deal of experience in media prep and central services from the runs. This schedule also provided sufficient time for agitator/mixing characterisation by MS&T personnel, also to reduce the risks of scale-up. MS&T staff also used lab scale chromatography to verify cell culture quality at harvest from a 15,000L bioreactor, with additional testing of all production support equipment and processes as required.
Scale-up risks were not limited to upstream processes. MedImmune needed to guarantee sufficient run-time on downstream equipment during shakedown runs. In particular, it was concerned about the scale-up to using 180cm chromatography columns. To complicate planning further, it did not want to incur the cost and time to repack columns. Thus, it limited shakedown runs in purification by requiring that only GMP materials passed through the columns. To mitigate this risk, the company implemented a number of water runs that enabled experience to be gained in transfer from buffer hold tanks through chromatography columns and to product hold tanks.
Though this shakedown methodology would reduce the risk of a failed PV run, it would have other collateral effects. First, beginning shakedown runs during ICQ increased the scheduling complexity for the job. This required daily co-ordination meetings to plan activities on every piece of equipment in the facility, forcing ICQ, Process Qualification (PQ), Preventative Maintenance (PM) and calibration activities to defer to shakedown activities.
The major impact was the need to drive GMP area release earlier in the project to support GMP column-packing. This decision required Environmental Monitoring Performance Qualification (EMPQ) to be moved up in the schedule. This approach benefited the overall process by allowing the project team to leverage shakedown activities to support cleaning validation, sterilisation validation, and mixing studies.
Most important, however, was the ability to reduce the risks to starting up the centrifuges. The centrifuges could not be tested effectively without using live cells and the shakedown methodology maximised the number of runs through to centrifuge to aid in start up and cleaning validation.
The shakedown process was even more successful than hoped. From the first vial thaw, the company successfully grew cells and made product that met all established metrics and criteria. To maximise the number of shakedown runs and to reduce the impact to schedule of a lost batch, the company thawed a new vial every week from the beginning of shakedown runs to the completion of PV runs.
MedImmune had planned time for problem resolution during each phase prior to moving to the subsequent phase. This time, however, it turned out not to be necessary. In fact, the company was able to combine phases two to four due to the success of its process characterisation. It had to defer some IOQ and PQ activities for seed bioreactors to accommodate the continuation of shakedown runs, but saw no decrease in performance in doing so. Despite the fact that the equipment wasn’t fully validated while it was performing shakedowns, it continued to have success.
From the first batch in the production bioreactors the viable cell density, cell growth and product titre closely matched the average results for more than 200 batches at Building 636 and 20 batches at the company’s contract manufacturing organisation.
Impressive downstream shakedown results were also achieved. The downstream group executed only two integrated water runs, one partial shakedown run (skipping a single chromatography step), and three complete shakedowns runs prior to the commencement of PV runs. In addition, the team successfully packed each of the three columns on the first try, despite having no previous experience in packing columns of this size.
The project team successfully completed more than 13 shakedown runs and three PV runs, and did so concurrent to on-going construction work. From the start of manufacturing in the facility, the product met all established process benchmarks at both medium and large scale, without a single contamination incident or lost batch.
