The US FDA Drug Supply Chain Security Act (DSCSA) is progressing towards establishing requirements for manufacturers and trading partners to have full interoperable electronic track and trace systems in place by November 2023.
The goal is to improve traceability of drugs by creating a consistent and efficient process for verifying and protecting the legitimacy of drug products at the unit level, as they move throughout the U.S. pharmaceutical supply chain.
DSCSA Implementation deadline: What needs to happen and by when?
The US DSCSA was enacted on Nov 2013 and since then we have seen a phased approach from the US FDA to implement the requirements. The upcoming deadline requires the partners to exchange all TI (Transaction Information), TH (Transaction History) and TS (Transaction Statement) in a secure, interoperable, and electronic manner. The TI must include the unique product identifier at the package level for all the packages included in the transaction. Also, a system needs to be in place to support the request for verification or investigation of suspects or illegitimate products.
The timeline below highlights the key steps for implementation over the period of 10 years:
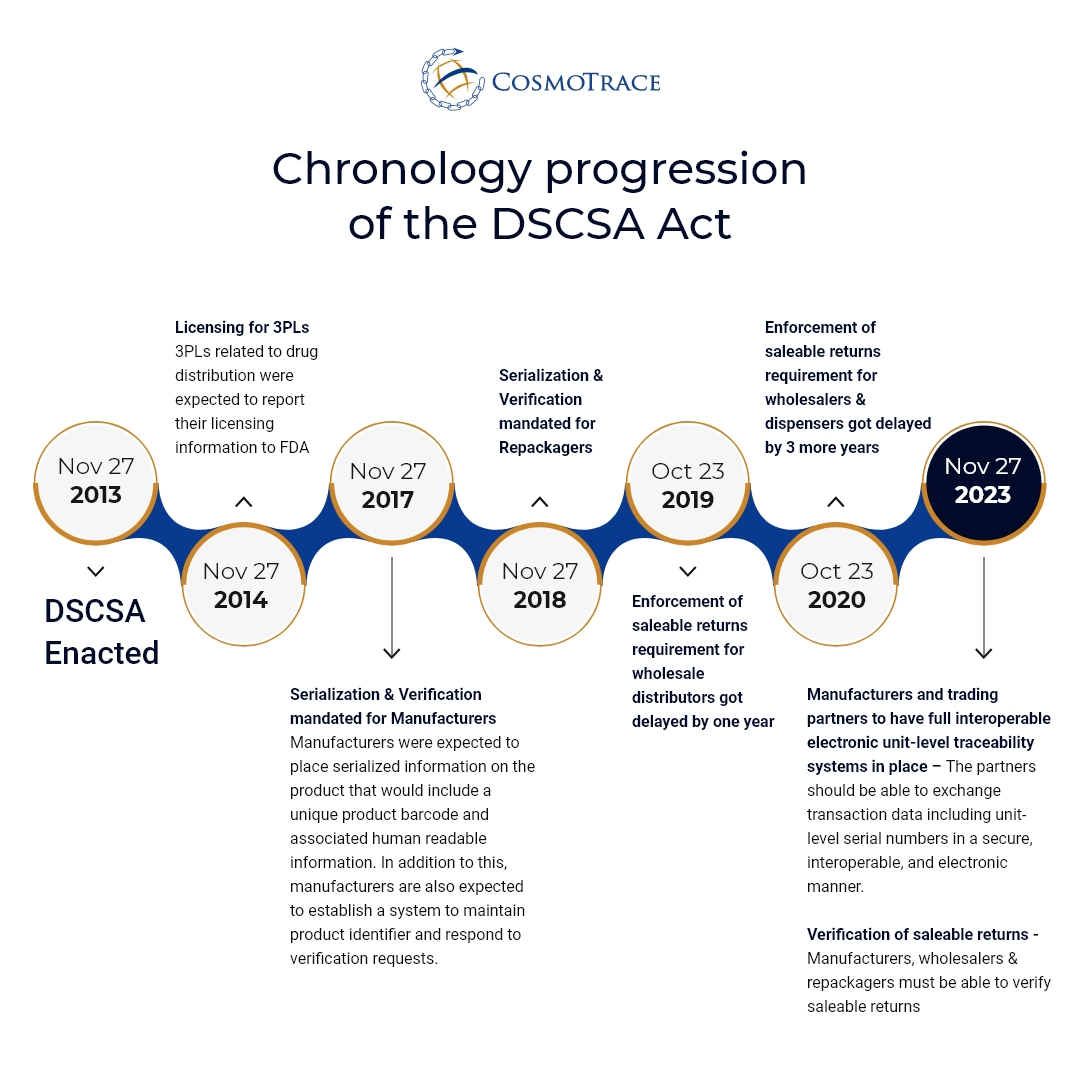
However, 3PLs are encouraging pharma companies to be ready by milestone date of Nov 2022.
Preparing for 2023 DSCSA deadline
Serialisation
Serialisation has been made mandatory by the FDA which requires all players in the secure drug supply chain to adhere to the traceability requirements. Which means that manufacturers, re-packagers, distributors, and dispensers must generate, authenticate, and verify the serial numbers for all products in the supply chain.
The Standardised Numeric Identifiers (SNI) for the serial numbers must be generated as per the FDA directive
- SNIs consist of the 20-character NDC (National Drug Code) and serial number.
- Apart from SNIs, batch/lot number, Global Trade Item Number (GTIN), and expiration date are also required.
- The smallest saleable unit needs to be packaged with a 2D Barcode Matrix with human-readable text.
Aggregation
Aggregation is the process of building a relationship between unique identifiers assigned to packaging containers. Shipping cases also require an SNI with a Serial Shipping Container Code (SSCC).
VRS (Verification Router Service)
To prevent counterfeiting of saleable returns, wholesalers need to ensure that saleable returns are verified before being introduced to the supply chain again and has an interoperable system that provides interoperable data exchange. This is achieved by initiating a verification request by the wholesaler to the manufacturer to verify the returned products and the manufacturer must provide a response within 24 hours. The verification router service enables real time exchange of this information between the parties.
3T Documentation
Transactional informationThis includes the Product Information, the following four items are part of the Product Information
- NDC: [insert product’s NDC]
- SERIAL: [insert product’s serial number]
- LOT: [insert product’s lot number]
- EXP: [insert product’s expiration date]
The DSCSA will continue to use The Electronic Product Code Information Services (EPCIS) standard for exchanges.
So, to summarise, the transactional information including the Product Information should contain.
- The name of the product
- Dosage and strength of the product
- NDC (National Drug Code)
- Serial Number
- Lot number
- Date of transaction
- Date of Shipment
- Date of Expiry
- Container information
- Number of containers
Transactional history
Complete outline of all the transactions the product has gone through in its journey right from the manufacturer to the dispenser which summarises the product's entire supply chain journey and has the required transaction information.
Transactional Statements
These statements identify if the seller:
- Is registered & authorised
- Has received the product from a registered and authorised party
- Did not purposefully change the transaction history
- Did not purposefully ship any ineligible, counterfeit, or suspicious product
- Has acknowledged the transaction statement and information from the previous seller in the supply chain
Disclaimer
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just one possible interpretation of information available in the public domain or through membership organisations, and that interpretation is subject to change. This information does not constitute legal advice. Users must refer to the source material for the complete requirements and form their own interpretation before making business decisions. Please use the references below to follow the updates at the source.
