Clearly, not all drugs are suitable for oral administration, which is the most straightforward route. Others, especially some biologics, often need to be delivered subcutaneously. This is one of the factors driving the rise of subcutaneous injection as a route of administration for drug delivery.
Many biologic drugs, including therapies for diabetes and autoimmune diseases, have been formulated for subcutaneous administration so that patients and carers can inject at home and help to manage their own condition.
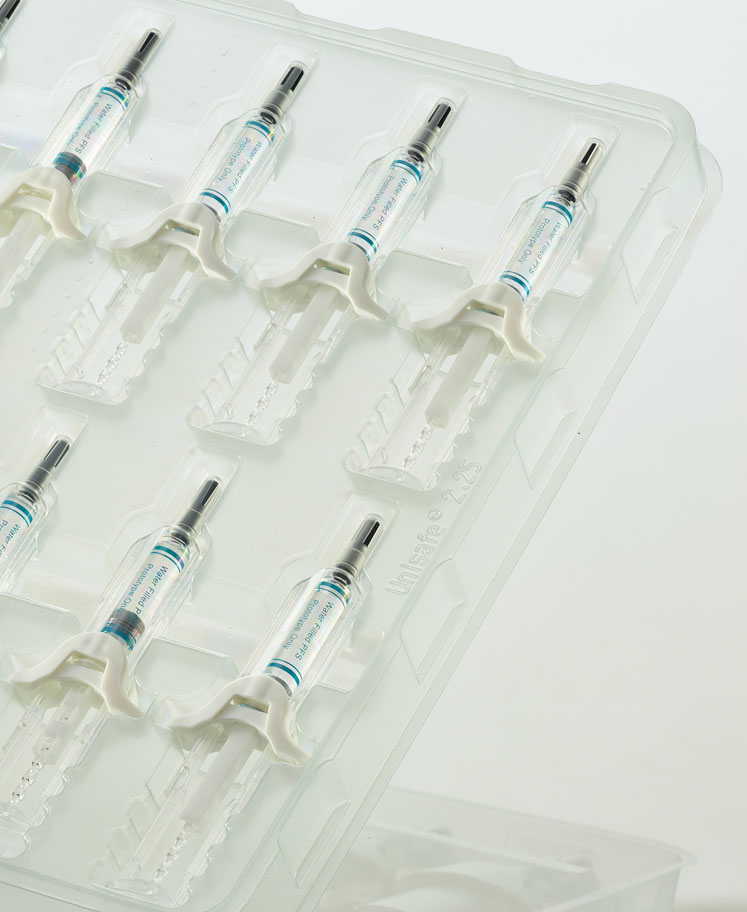
These market dynamics have led to changes in the design and function of drug delivery devices. Typically, standard prefilled syringes and safety devices hold 1 mL fill volumes and accommodate drugs with a viscosity (usually) less than 10 cP.
However, these devices may no longer be appropriate for some of the new biologics now being developed, which tend to be higher in volume and viscosity. Without prefilled syringes and drug delivery devices adapted to larger volumes, some treatments may require more frequent dosing and, potentially, repeated visits from healthcare practitioners or hospital visits.
The case for larger injection volumes
Designing injection devices for volumes greater than 1 mL introduces new complexities, risks and safety concerns during drug delivery. But addressing these issues has the potential to produce a number of benefits for both patients and pharmaceutical companies.
If patients can self-administer larger volumes — up to 2.25 mL — they may need to inject less frequently, which is likely to improve compliance as well as quality of life. From a healthcare perspective, fewer injections and better therapy adherence may translate to improved outcomes and, ultimately, cost savings.
There is a clear case, therefore, to develop devices that deliver higher-volume and potentially higher-viscosity drugs, especially as biological actives are becoming increasingly effective and can dramatically improve treatment outcomes for chronic conditions.
Additionally, new device technologies may provide businesses with a critical differentiator in a competitive environment as patents on branded biologics continue to expire and allow less expensive biosimilars to compete for a share of the market.
Adapting drug delivery device design
Let’s take a closer look at the challenges of developing drug delivery devices for higher volumes and viscosities … and solutions to help address these challenges.
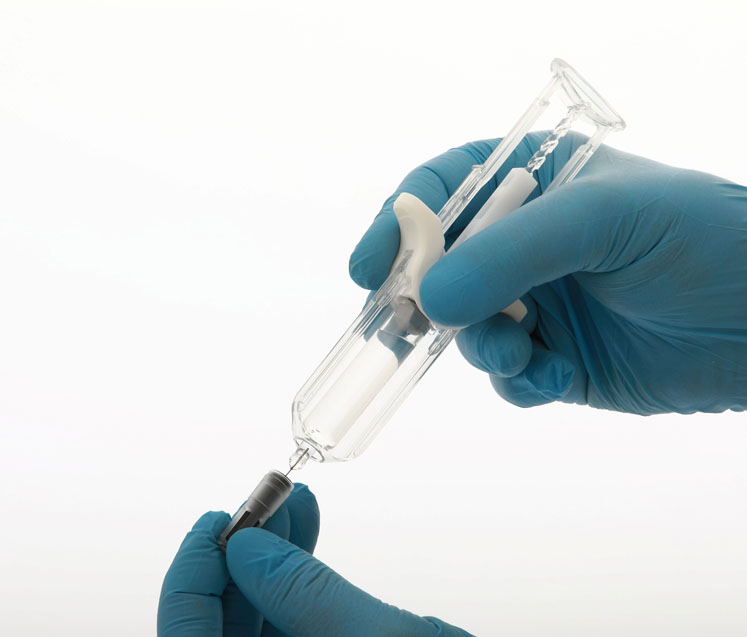
Injection force: As biological drugs are made of large, complex protein molecules, they tend to be more viscous than chemically based medicines. High viscosity drugs can make manual injection with a prefilled syringe challenging, especially for patients with limited strength or dexterity who may struggle to apply the necessary force or hold the injector in place for the duration required.
Prefilled safety devices should permit function within reasonable parameters for injection force and duration: injection forces should not exceed 10 N, whereas the duration should not be any longer than 10–15 seconds. If device design exceeds these limits, certain patients may not be able to fully deliver the drug dosage, which means that compliance and efficacy could be impacted.
Manufacturers should therefore ensure that they create a safety syringe that is comfortable to hold and intuitive to use. In addition, the plunger must be easy to fully depress without undue force.
To enable this, human factor testing must take place within different user groups to ensure that all patient demographics and needs are taken into consideration and any risks are mitigated as far as possible. Prefilled safety syringe devices are highly suitable for biologics as they allow patients to control injection speed, force and, to an extent, the potential level of pain.
They also habitually integrate critical safety features to protect users from needlestick injuries. Prefilled syringes containing biologics are typically refrigerated for transport and storage, which increases drug viscosity. For this reason, and to minimise discomfort, they must be allowed to reach room temperature before use.
Needle size: For subcutaneous injections, 29G, 27G or even 25G needles are most commonly used. Larger diameter needles make it easier to deliver highly viscous formulations, but could be painful for patients and might lead them to remove the device before completing injection; this creates the potential for drug spillage or so-called “wet injections.”
Switching to needles with larger diameters is also likely to increase the chance of pain during injection. To help resolve this problem, thin-wall needles have been developed and are typically used for more viscous drugs.
Dosage volumes: Dosage volumes for biologics delivered subcutaneously are relatively small — up to 2.25 mL as mentioned above — so it is essential that the full dose is delivered for the patient to receive maximum benefit.
To support this, patients should be able to clearly see the full contents of the syringe both before administration to check for drug colour and clarity and afterwards to ensure full dose delivery.
It is highly recommended that the design also prevents removal of the syringe plunger as this can result in leakage or spillage of expensive biologics during transport or use, as well as potentially creating a risk of tampering.
Regulatory approval: When asked about the chief obstacles associated with getting patient-centric injectable combination products to market, pharmaceutical executives ranked regulatory concerns as their biggest challenge.1
Delivery devices are becoming more sophisticated to accommodate larger volumes and higher viscosities, as discussed above, but meeting key ease-of-use requirements will be important for combination products to achieve regulatory approval. Moreover, the emergence of new technologies such as connected devices has led to new guidelines and standards, which businesses must then keep up with.
As pharmaceutical businesses must secure approval for the drug itself, and for the drug-device combination product in the US, they must consider every element of the final product from the beginning of the design process. If the drug delivery device fails usability and safety testing, this will result in delays in getting the combination product to market.
Similarly, the compatibility of the primary container with the chosen delivery device should be verified as early as possible to prevent unnecessary delays and associated costs. Ideally, the combination product should be designed for easy assembly and manufacture at scale, as well as being simple and intuitive for the patient to use.
Innovating for improved delivery
Adapting subcutaneous drug delivery devices for higher viscosity and higher volume drugs allows patients to benefit from new therapies without a negative impact on their daily lives. Manufacturers must therefore strive for innovation while continuing to maintain comfort and safety for end users.
In terms of the formulation itself, some pharmaceutical companies are looking into using excipients with biologics to improve subcutaneous absorption and dispersion (around a third of respondents in the survey mentioned above), whereas others are more cautious, perhaps because excipients may alter the characteristics of the original formulation.
Regarding drug delivery, wearable injectors can administer volumes greater than 2 mL, so highly concentrated drugs can be diluted into larger volumes and administered subcutaneously during longer periods.
However, despite the potential benefits of safe and easy-to-use wearable injectors, particularly for diabetes patients, this method of administration is still an under-developed segment in other disease areas.
Some patients may be hesitant to adopt wearable technologies already available, perhaps as they need to be kept in place on the injection site for a period of time or because they may impede activities such as showering or swimming.
This makes it clear that failure to consider patient needs, attitudes and behaviours during the delivery device design process may create obstacles to achieving improved therapy adherence. Selecting an appropriate drug delivery device is invaluable to obtain maximum benefit from new and emerging biotherapeutics.
