Every small molecule drug discovery project starts with deciding how to identify the starting “hit” molecule(s), which will subsequently be optimised to produce the final marketed drug.
The selection of this hit matter is key; progressing an inferior hit molecule may lead to pursuing compounds with inadequate potency, selectivity and pharmacokinetic profiles. Hence, any clinical candidate derived from this series is likely to end in failure in the clinic.
During the last few decades, hit-finding strategies have predominantly involved established platforms such as high-throughput (HTS) and computational virtual screening.
However, the more recent concept of fragment-based drug discovery (FBDD) is now gaining traction … and more companies are now adopting this as part of their hit discovery engine.
What is fragment-based drug discovery (FBDD)?
Fragments are small organic molecules that are low in molecular weight and typically bind weakly (in the µM to mM range) to the biological target of interest.
Medicinal chemists develop these fragments to deliver a lead molecule(s) with higher affinity and, potentially, improved selectivity compared with that obtained by HTS or virtual screening campaigns. The screening of low molecular weight fragment libraries has many advantages compared with more traditional lead-like libraries.
The fragment hits obtained often have higher ligand efficiency (a measure of the binding energy per atom in the molecule used to bind to the protein target) compared with hits derived from other platforms, which tend to contain superfluous atoms/moieties not required for potency.
In addition, the hydrophilic nature of the fragment hits improves their physicochemical properties and increases the chance of developing a candidate drug more efficiently.
One of the advantages of FBDD is the relatively low cost of delivering hit compounds. This is because of the somewhat low number (a few thousand) of compounds required for the initial screening campaign compared with HTS (for which between 25,000 to more than one million are typically screened).
The budget available for “hit identification” campaigns can be vastly different for an established large pharma organisation compared with a small biotech and, therefore, HTS may not be an option when the budget is limited.
Another advantage is time. A traditional HTS programme can take 6 months or longer as it’s necessary to both establish a technique that’s suitable for screening tens of thousands of compounds and to then conduct the actual campaign.
In comparison, FBDD projects can take as little as 6 weeks to do, including biophysical assay optimisation and the screening of a few thousand fragments.
Hit identification
Domainex, a fully integrated drug discovery CRO, offers a range of hit discovery options to its clients who do not have the capabilities or experience in this area.
The company’s hit identification services include both virtual screening (LeadBuilder) and the FBDD platform (FragmentBuilder). The latter includes a suite of services to advance FBDD projects, including
- protein expression and characterisation
- assay development and screening across several technology platforms, including microscale thermophoresis (MST), grated coupled Interferometry (GCI) — a technique that’s similar to surface plasmon resonance (SPR) and homogeneous time resolved fluorescence (HTRF)
- access to a large fragment library
- orthogonal techniques for hit confirmation
- X-ray crystallography to determine the location of binding sites and identify fragment expansion opportunities.
Medicinal chemistry to progress the hits
FBDD platforms are the route of choice compared with a potentially time-consuming and expensive HTS campaign (Figure 1). They deliver greater insight, more options for optimisation and, potentially, uncover novel modes of binding or allosteric inhibitors.
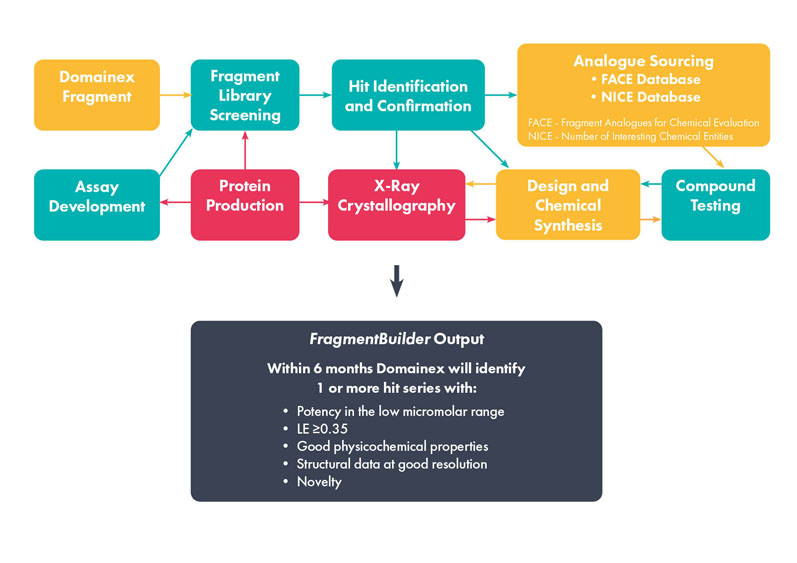
Figure 1: Overview of the FragmentBuilder process
In-house fragment library
When undertaking a FBDD approach, it is essential that the fragment library used has been carefully curated from multiple suppliers to provide a diverse collection of fragments with excellent coverage of the bioactive fragment space.
Domainex’s library contains more than 1000 fragments and has the following features:
- a multiparameter scoring function that’s used to select compounds in the optimal fragment space
- molecular fingerprints that are used to compare interaction points, shape and dipoles with ChEMBL fragments to ensure the library provides adequate coverage of the bioactive space
- access to sp3-rich fragments from SpiroChem provides original starting points, enabling rapid access to novel chemical space
- all compounds are soluble at 1 mM in 1% DMSO.
Many compounds made by medicinal chemists have a high degree of sp2 centres and a small number of sp3 centres. This can result in flat, insoluble molecules.
To some extent, this reflects target preferences, but it is also a reflection of the narrow range of chemical reactions that medicinal chemists like to employ.
Domainex’s fragment library shows a good balance of compounds with high, intermediate and low sp3 content and therefore allows target preference to guide hit selection rather than biasing it towards flat molecules.
Fragments typically have low molecular weight, a limited number of hydrogen bonding groups and rotatable bonds, and a balance of lipophilicity (Figure 2).
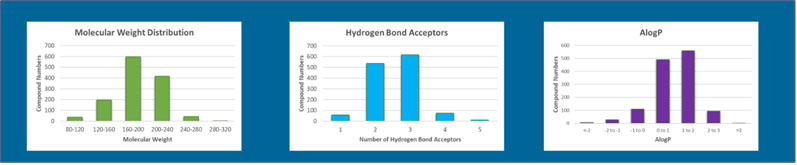
Figure 2: Some molecular and physiochemical properties of the Domainex fragment library
Fragment screening
Screening is a key component of any FBDD platform. To achieve the best results, it should be used with methods that have a good track record of being able to detect the relatively weak binding of fragments.
Typically, we aim to deploy either the Creoptix AG WAVEdelta platform, which uses GCI, or NanoTemper thermo-optical instruments (Dianthus NT.23PicoDuo and Monolith NT.Automated), which use the techniques of MST and temperature related intensity change (TRIC) to detect ligand binding (Figure 3).
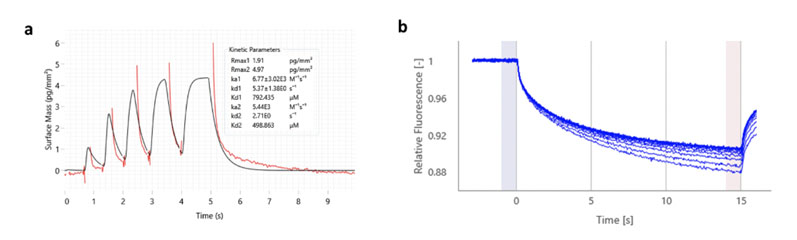
Figure 3: waveRAPID kinetic data obtained using the Creoptix WAVEdelta system for a G9a fragment hit (Frg331); a KD value of 499 µM was obtained (a); MST data for the same fragment hit (a KD value of 460 µM was obtained using this technique)
A range of alternative biophysical screening methods can be used if required. In addition, HTRF has also been found to be a robust and very useful technique in some cases.
Fragment hit expansion
Applying a philosophy of “every compound counts” means that molecules can be designed with all the relevant properties in mind, including biological activity, selectivity, pharmacokinetic and toxicology properties to produce developable, patentable compounds and minimise late-stage attrition.
As such, in addition to traditional synthesis and array chemistry, Domainex has invested in flow chemistry using the Vapourtec E-Series flow reactor. Our synthetic and medicinal chemists can incorporate flow chemistry into the “design-make-test” cycles to rapidly generate structure activity relationships (SARs) and improve fragment potency.
For those that are unfamiliar with flow chemistry techniques, the technology utilises a number of channels — or, simply put, tubing — to conduct a reaction in a continuous stream rather than in a traditional round-bottomed flask!
Flow reactors provide chemists with exquisite control of reaction parameters, meaning that the reactivity of species can be enhanced or new reactions identified.1
Application success
During a recent a project with APEIRON Biologics to identify hits against variants of the E3 ligase Cbl-b., a suite of biophysical techniques was deployed to determine the binding affinity, mechanism and selectivity of promising compounds … and has now progressed into the lead optimisation stage.
Peter Llewellyn-Davies, CEO of APEIRON Biologics, commented: “Finding the right research partner for the development of our highly innovative immune-oncology therapeutics is essential for fast, efficient and, ultimately, successful results."
"We are delighted to expand our partnership with Domainex for our Cbl-b targeted APN431 project and quickly move to the next stage of its development. The company provides considerable know-how in state-of-the-art structure-based medicinal chemistry and assay development to help us achieve our ambitious goals."
"With our APN431 small molecule programme, we have an additional approach to target Cbl-b, next to our autologous cell therapy programme, APN401, which is currently in clinical Phase I development.”
Fragment-based drug discovery programmes demonstrate the benefits of intelligent, integrated and collaborative approaches to drug discovery.
Whilst technology has undoubtedly progressed our pipelines considerably in recent years, targeted and combined use of these new technologies will advance the development of medicines considerably faster than quantity-based screening programmes alone.
In a world of streamlined research teams, contract research organisations clearly have a significant role to play in the efficient delivery of new and effective medicines.
The importance of this rapid and versatile nature of research collaborations has never been more evident than during this global pandemic, benefiting organisations and patients alike.
Reference
1. https://pubs.acs.org/doi/pdf/10.1021/acs.chemrev.7b00183.
