SARS-CoV-2 had all the marks of a similar endemic-level virus – until it didn’t. Now, the whole world is watching as biopharmaceutical companies work to accelerate vaccine development and production to an unprecedented scale.
Jim Sanford, Dr Sade Mokuolu and Peter Birch from Watson-Marlow Fluid Technology Group (WMFTG) discuss how the biopharmaceutical community is coming together to solve this global challenge. Do we have the technology, skills and resources to respond to COVID-19? And what will this mean for vaccine development in the future?
The race to make a billion doses
Developing vaccines is crucial to combating existing and novel diseases. A World Health Organization (WHO) report based on 2013 data found that, globally, immunisation averts two to three million deaths every year in all age ranges. Vaccines must be produced in large batches at a low cost per dose to offer broad and effective protection.
From drug discovery to biomanufacturing, technology innovation continues to thrive with advancements in process intensification, cost reduction and risk mitigation initiatives.
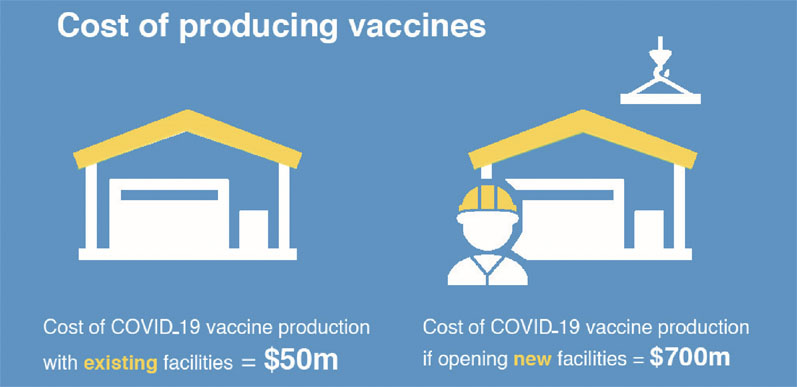
Gavi, the Global Vaccine Alliance, estimates that the cost of producing hundreds of millions of doses of a COVID-19 vaccine would run into $50 million for those companies that already have facilities and $700 million for those who are opening new ones. The alliance has already reallocated $200 million in funds as an immediate response to this crisis.
As COVID-19 continues to affect communities throughout the world, there is collective hope that a vaccine can be developed and subsequently manufactured. With more than 100 COVID-19 vaccine candidates in research stages and others in promising clinical trials, it seems likely that a vaccine will be developed.
But is the biggest problem still to come? Once a vaccine is proved to be safe and effective, how will the industry mass produce these vaccines to such a demanding schedule on a global scale?
Acceleration starts in research
We’ve already seen research and development (R&D) on an unprecedented scale. Processes that would usually take years have been accelerated to just months as researchers use existing platform technology to expedite development … with some level of assurance of efficacy and safety extrapolated from other trials. Now it’s time to take this approach into the manufacturing space.
R&D teams focus on using their skills and understanding to create a therapy; the link between researchers and the manufacturing process has never been so important. In recent years, vaccine production has moved to a continuous bioprocessing model and we’ve seen process intensification on a scale-out — rather than a scale-up — basis.
Put simply, the bioprocessing equipment used in research stages can now be used for commercial manufacturing. When time is short, process design engineers and researchers need to work together to ensure that the vaccine can be replicated reliably at scale for clinical trials and then again at an even greater scale for mass production.
Production won’t take place in just one location; multiple vaccine manufacturers will need to support product demand and, for these vaccines to be available at scale, they will need to be produced in numerous locations all around the world. Processes and equipment need to be standardised and this activity needs to start at the R&D stage. Each biocontainer, clamp and tube assembly needs to have an equivalent for use at scale.
Never has research been so focused on a singular, tangible goal. Although there is a fascinating exploration into novel vaccine technologies, the current requirement is for tried and tested techniques, performed and understood by skilled teams.
Manufacturers such as WMFTG have a role to play in this process. Our teams help to form a link between R&D and process engineer groups and we assist in ensuring that our equipment is used appropriately in the different stages of vaccine development.
Scalable versions are manufactured using the same materials and processes, reducing additional validation steps and accelerating the progression from bench to production.
Reproducibility opens the black box
Single-use bioprocessing systems enable regional production on a global scale. Biopharmaceuticals and their contract manufacturing organisations (CMOs) are already creating vaccine production hubs at an extraordinary level using single-use technology that ensures drug product consistency.
Once manufacturing systems and fluid pathways are tried and tested, and can demonstrate a safe and efficacious vaccine, this system has the opportunity to be replicated anywhere in the world. The process becomes the most important proof of safety and efficacy.
Vaccines produced in the millions can’t all be tested and bioprocessing — by its very nature — is subject to the behaviour of individual cells and the black box of molecular interactions. However, the process can be controlled and replicated assuredly!
Suppliers of biological manufacturers continue to design innovative bioprocessing systems that deliver reproducibility as well as process flexibility, cost control, risk mitigation, security of supply and sustainability.
Equipment versatility also plays a role here. Fewer processes also mean quicker upskilling for operators.
Outsourcing quality for regulatory approval
By using tried and tested bioprocessing technology, biopharmaceutical companies can increase production by outsourcing to CMOs. But outsourcing doesn’t stop with production; it can extend to quality too. Equipment validation is crucial in an effective bioprocessing pathway. To ensure the highest quality, the process must demonstrate reproducibility and safety. To do this, each piece of equipment needs to be qualified and validated.
Single-use technology holds the key to regional production
Single-use technology has increased the regionalisation of drug manufacturing. Drug products such as vaccines are more likely to be manufactured in the regions where they are required, providing more cost-effective products without the reduction of efficacy caused by potential logistics issues impacting product quality.
In these new regional facilities, flexibility is key. As such, the “ballroom” concept and other manufacturing techniques can be employed to give highly functional multiple product facilities. The ballroom concept of manufacturing can use non-classified spaces because of the integrity of sterile, single-use bioprocessing equipment.
With this approach, developing nations can access the same manufacturing techniques as those in developed countries, such as in the USA, Western Europe and China. Process validation can be largely location independent, assuming that the conditions of the manufacturing protocols are met.
A “shopping list” of single-use equipment and consumable parts can be sourced locally through manufacturer distribution and supply networks. Skilled staff can be employed locally and trained to ensure safe and compliant production.
