There are many types of cancer therapies, with surgery and radiotherapy often being offered as first choices. These are then followed by chemotherapy to stop or slow down cell growth and, hopefully, prevent the disease from returning after surgery. In recent years, research has increasingly led to alternative cancer therapies that employ the human immune system itself.
Antibody drug conjugates (ADCs), which use antibodies to send cancer-killing molecules directly to where they are needed, remain a key area of focused research. ADCs combine the potency of small molecules with the selectivity of cancer-specific antibodies.
ADC innovation continues apace, driven by developments in each of their core molecular components. Given they comprise a small molecule linked to an antibody, the effective production of these medicines is complex and requires experience in both biologics and small molecules development.
Range of immunotherapies to treat cancer
Immunotherapies work by activating or suppressing the patient’s immune system. These medicines may use one of a range of mechanisms of action in the treatment of patients:
- General activation of the immune system: cytokines, Bacillus Calmette-Guerin (a weakened bacterial form) or immunomodulatory drugs activate the immune system in a general way that can lead to the removal of specific cancer cells.
- Block molecules that the cancer cells need to grow: Bevacizumab (Avastin) binds to vascular endothelial growth factor (VEGF), thereby reducing vascular growth and preventing blood (oxygen) reaching the cells.
- Flag the cancer cells for destruction: Rituximab (Rituxan) binds to CD20 on the cancer cell, causing the immune system to destroy the cancer cells.
- Deliver drugs, toxins or radioactive particles: ADCs are internalised into the cancer cell by binding to specific receptors on the cancer cell, thus delivering the drug, toxin or radioactive particles into the cell, causing cell death. Although the mode of action does not directly affect the patient’s immune system, immunoconjugates such as ADCs exploit the selectivity of antibodies as a delivery system for cytotoxic compounds.
Immunotherapies may be designated as “activation” therapies if they elicit or amplify an immune response or “suppression” therapies when the immune response is reduced or suppressed. Much research is being done globally to increase the precision of these therapies.1
Therapies based on the “delivery” principle ferry payloads to cancer cells and rely on protein structures related to those that are naturally produced by our immune system.
The rise of antibody drug conjugates
Antibody drug conjugates are a good example of this last mechanism of “delivering” toxins to specific cancer cells within the body. The antibody moiety specifically targets proteins that typically control how cancer cells grow, divide and spread; the toxin moiety specifically interferes with the cell division process — either via disrupting the microtubule machinery or by inducing DNA damage. The process ultimately leads to cell death.
Since the first ADCs hit the market in the early 2010s, researchers have focused on the efficacy of these products while minimising their potential side-effects. Innovations in ADC design and effectiveness have followed the three main components of these medicines: antibody engineering, toxin selection and linker design.
Antibody engineering
Improving the binding selectivity to cancer receptors has improved specific cancer type targeting and minimised their effect on benign cells. In addition, finding the optimal place to connect the linker (structural integrity) and increasing the number of toxins per ADC have been recent areas of progress.
Although initial ADCs typically had two to four toxins per antibody, known as the Drug-Antibody-Ratio (DAR), more recent development by Daiichi-Sankyo has led to the Enhertu ADC that carries up to eight toxin moieties on one antibody.
Toxin selection
The first ADCs to reach the market were equipped with Auristatin, Maytansine or Calicheamicin antineoplastic (anticancer) payloads. This toxic cargo was designed to interfere with the microtubule machinery or to cause breaks in DNA’s double-strand structure. When the cell division cycle cannot be completed, the cancer cells will die. The following compound classes/mechanisms are known to achieve this result (Figure 1):
- DNA alkylating agents (cisplatin, calicheamicin)
- antimetabolites (methotrexate, which blocks dihydrofolate reductase [DHFR])
- antimicrotubule agents (plant-derived chemicals that prevent microtubule function such as Vinca alkaloids, Taxol, Auristatin and Maytansine)
- topoisomerase inhibitors (influencing topoisomerase I and II enzymes that support double-helix unwinding during replication, such as irinotecan and doxorubicin)
- amanitins block RNA polymerase and are seen by some in the field as attractive based on their different mechanism of action.
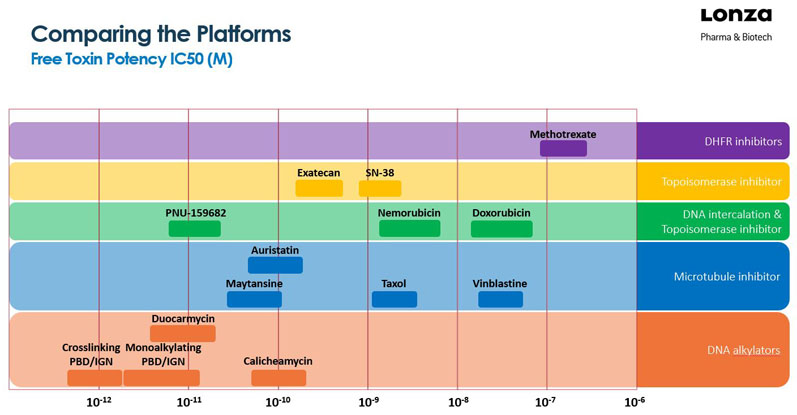
Figure 1: Overview of ADC payload toxins (with some chemotherapies for reference)
More toxic payloads, such as pyrrolobenzodiazepines (PBDs), were expected to generate better results and several companies brought examples into the clinic. However, many of these products failed in clinical studies, partially because of an unstable linker, which released free toxin in the bloodstream and induced severe side-effects.
Additionally, the high hydrophobicity of PBDs caused aggregation problems of the ADC itself.2 Currently, there are several PBD-based ADCs undergoing clinical trials.
Interestingly, recently approved ADCs seem to underline a trend towards less toxic payloads. Daiichi-Sankyo’s new Enhertu ADC uses their proprietary DXd platform (an exatecan and a more potent analogue of SN-38). Similarly, Immunomedics’ Trodelvy ADC carries the SN-38 payload, which is the active metabolite of the systemic chemotherapy drug irinotecan.
Some ADCs in development carry a non-toxic photosensitiser payload instead of a toxic small molecule. Upon irradiation by near-infrared light, the sensitiser creates holes in the membrane of the tumour cells that induces cell death. Even when these photoimmunotherapeutics (PITs) are distributed throughout the body, they are harmless.
Such photoimmunotherapy opens great treatment options when surgery cannot access the diseased areas (such as head, brain and neck tumours) and represent a further step in precision medicine. The first commercially approved example in this category of products is Akalux (cetuximab saratolacan sodium) by Rakuten Medical.3
Linker design
The linker has been a key aspect of ADC research in recent years; the biggest challenge being that the linker must break and release its payload at the right place … and not before. When the linker is too strong, it will maintain the ADC’s integrity inside the patient (desired), but possibly not release the payload once it’s internalised within the cancer cell.
This reduces its efficacy and ability to kill the tumour (undesired). When the linker is too weak, it may release its cargo before reaching the tumour cells, thus reducing its efficacy and increasing free payload toxicity side-effects.
Increasingly, ADC linkers are designed to be susceptible to specific enzymes that are present at increased levels within tumour cells (such as the tetrapeptide-based linker used by Daiichi-Sankyo in Enhertu). In addition, an appropriate linker design can resolve hydrophobicity issues, as described above for the PBD toxins.4
As organisations still need their products to be unique, this area may be ripe for future innovation.
There are some payloads, such as DM1 (a maytansinoid), which tolerate chemical modification on their pharmaceutically active core and can therefore be incorporated into an ADC using non-cleavable linkers. In this case, the active species is released after internalisation and digestion of the ADC as a payload-linker-amino acid derivative.
Outlook for future ADC development
The number of approved ADC products has risen from three to nine in the past 4 years alone. Many technical challenges that became evident during early development and clinical trials have been resolved, and innovations continue apace.
As a result, the complete understanding of antibody drug conjugates has shifted from either antibody engineering, toxin selection or linker design to a more integrated perspective on these molecules.
However, to drive development and manufacturing excellence regarding ADCs, these projects are often outsourced to a contract development and manufacturing organisation (CDMO). CDMOs with a skillset covering both the biologics as well as the chemical aspects of the programme will be an advantageous partner; their combined perspective may actively contribute to timeline reductions during the programme.
In addition, overcoming the manufacturing challenges associated with the antibody (sterility), toxin (HPAPI and containment) and linker (chemical expertise), as well as the ADC itself (all of the above), will be key to helping pharma and biotech innovators bring the next products to the market and to patients.
References
- www.cancer.gov/about-cancer/treatment/types.
- www.pharmtech.com/view/adc-targets-fail-because-aggregation-problems.
- https://rakuten-med.com/us/news/press-releases/2020/09/25/7442.
- https://cen.acs.org/content/cen/articles/98/i14/new-generation-antibody-drug-conjugates.html.

