Poor permeability of pharmaceutical actives prevents many drugs from reaching the market. David Gschneidner, Emisphere Technologies, highlights a technology that can improve the delivery of water-soluble compounds
The challenge of developing oral delivery for therapeutic molecules lacking optimal properties has stimulated much research in the pharmaceutical industry. Common problems that are continually faced in developing oral dosage forms of active pharmaceutical ingredients (APIs) include very low aqueous solubility, high polarity or high molecular weight, resulting in a lack of permeability, and degradation due to high acidity or enzymatic activity.
Over the years, chemists and pharmaceutical scientists have been able to massage some formulations to overcome these issues to develop oral products. On the other hand, many molecules have been limited to parenteral dosing or, in some cases, shelved because they did not have the ‘druglike’ properties to be orally absorbed.
In recent years, the pharmaceutical industry has seen a shift towards a greater emphasis on biologics as drugs. Biologics are usually more specific in their targeting than small molecules, resulting in fewer safety issues. These peptides, proteins, oligosaccharides and nucleic acid derivatives are more polar, often readily degraded in the gut and typically much larger than small molecule drugs. The challenges of making these biologics orally available have been daunting, but much progress has been made.
addressing permeability
Emisphere has developed the Eligen Technology to address specifically drug delivery in which permeability is deficient. The company’s approach is to utilise small molecules as drug delivery agents for polar molecules that are not normally permeable to epithelial membranes. The technology has proven to be effective in achieving therapeutic levels via oral delivery of peptides,1 oligosaccharides,2 small polar molecules3 and even metals.4
These delivery agents (also known as carriers) have been found to perform a number of functions that, together, result in the increased bioavailability of drugs. The carriers are able to form weak non-covalent, non-specific interactions with the API of interest. These interactions cause the API to become more hydrophobic, perhaps due to replacement of the waters of hydration or by causing a change to a more lipophilic conformation. In addition, it has been shown that the presence of the delivery agents slows degradation of peptides in the gastrointestinal environment.
Figure 2 shows the different possible mechanisms of transport of molecules from the gastrointestinal tract to the bloodstream. Emisphere has demonstrated that for Eligen Technology the absorption occurs by passive transcellular transport.5
The integrity of the tight junctions has been shown not to be disrupted, making it clear that the mechanism of transport is not paracellular in nature. Also the process has been demonstrated not to utilise adenosine triphosphate (ATP) and thus does not occur via an active transport mechanism.
Additionally, in a caco-2 cell model using a fluorescently labelled drug such as insulin, the drug can, in fact, be seen to be transported into the caco-2 cells. The fact that transport occurs through the epithelial cells and not around them means that concerns about unwanted molecules or pathogens getting through into the bloodstream are minimised. Following transport there is no disruption of the membrane or the internal components of the cell and no permanent damage to the membrane.
Once the delivery agents have completed their job to transport the API to the blood stream, the delivery agents readily separate from the drug molecules (due to the weak interactions and the dilution due to the blood). Studies have shown that the therapeutic molecules are unchanged following transport and are fully pharmacologically active.6
Eligen Technology works to alter the absorption phase, affecting the rate and extent. The elimination phase is usually not affected. In most cases the absorption is quite rapid with Tmax typically within an hour of dosing and can even occur in 15–30min. An example of a typical pharmacokinetic profile is shown in Figure 3 in which vitamin B12 has been given to healthy volunteers, either as the Eligen formulation or as a commercial supplement of the same strength. With Eligen, Cmax was increased 10-fold and the AUC was 240% higher than with the commercial B12 tablet. In addition, Tmax occurred in just 30 minutes with Eligen B12 compared with 6.8 hours with normal absorption.
Emisphere has a library of 4,000 carriers from which it can select. The delivery agents are readily synthesised in a few short steps and are amenable to scale-up to kilo and even metric ton quantities. The carriers have been designed to be pharmacologically inactive and have been found not to accumulate in any organs. One of Emisphere’s carriers, SNAC, achieved GRAS (generally recognised as safe) status in the US in 2009 and thus is considered safe for use in supplements and foods. Selection of delivery agents for a particular drug is accomplished by matching the two based on Emisphere’s experience with more than 60 different drugs.
Unlike many other oral drug delivery technologies, with Eligen Technology, the formulation and manufacture of the final dosage forms are simple processes that are relatively easy to scale up. Typical pharmaceutical solid dosage forms (i.e. tablets and capsules) can be manufactured with standard pharmaceutical processing equipment. The delivery agents are incorporated into the blend with the API using well known processing techniques, other excipients are added and the final dosage form is obtained using common methodologies.
drug applications
In the past, Eligen Technology has been used successfully to improve oral delivery of small hydrophilic drugs such as cromolyn and bisphosphonates. Recently, work has focused on an improved cyanocobalamin (B12) formulation directed toward achieving repletion of active B12 in B12-deficient individuals. Low levels of B12 can be the result of a lack of the vitamin in the diet (such as for vegetarians), but are most likely to occur because of deficiencies in an individual’s ability to absorb B12 through the natural intricate mechanism (acid in the stomach is needed to release the B12 found in foods and B12 absorption is facilitated by a protein, intrinsic factor, at a particular location in the small intestine).
Conditions resulting in reduced stomach acidity (such as long term use of proton pump inhibitors or age-related stomach atrophy) or gastrointestinal disturbances (such as bariatric surgery, Crohn’s disease or celiac disease) can lead to B12 deficiency.
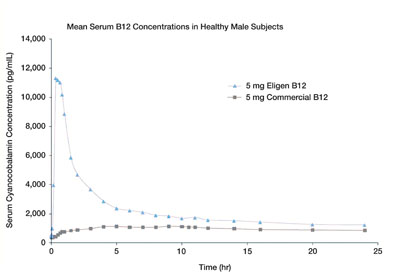
Fig. 3: Pharmacokinetic profile comparing Eligen B12 and commercial B12 tablets of a single 5mg dose in healthy male subjects
In a study7 completed last year in which B12 deficient patients were either given a typical B12 injection regimen for 12 weeks (5 injections of 1000µg over the first 15 days and then one each at 21, 30, 60 and 90 days) or 1000µg of Eligen B12 as a daily pill, every individual in the study achieved rapid repletion of active B12 within 15 days (the first time point in the study) whether on injection or the oral tablet. Furthermore, all participants in the study had B12 levels that continued to be at normal levels through to the end of the study. This performance places the Eligen B12 formulation on par with the standard regimen of frequent B12 injections, without the extra cost and inconvenience of drug injections.
Much work over the years has focused on developing Eligen Technology as a method to accomplish the goal of the oral delivery of therapeutic peptides and proteins. Emisphere has had a partnership with Novartis going back 12 years for the development of a number of oral peptide products. Three Phase III trials of salmon calcitonin have been ongoing for osteoporosis and osteoarthritis (one of the osteoarthritis trials has been completed and the osteoporosis trial is scheduled to be complete later this year). More information about these trials is expected later in 2011.
In addition, Novartis is continuing to explore oral delivery of parathyroid hormone (PTH) with a Phase I study begun in the spring of 2010.
In the field of diabetes, Emisphere has been engaged in a partnership with Novo Nordisk since 2008. Last year Novo Nordisk moved into Phase I with one of its GLP-1 analogues using the Eligen Technology with results expected later this year. In December 2010, Novo Nordisk expanded its relationship with Emisphere by licensing the technology for the oral delivery of its insulins.
In addition to improving the amount absorbed, Eligen Technology usually features fast absorption, which can be an advantage for certain therapeutics even in cases where an increase in oral bioavailability is not needed. For pain medications, the time to reach therapeutic levels is very important. In a preclinical study, Emisphere was able to demonstrate a significant decrease in the Tmax for the migraine medication naratriptan (see Figure 4). In this study8 the Tmax was halved to about 15 minutes by the use of Eligen Technology with naratriptan.
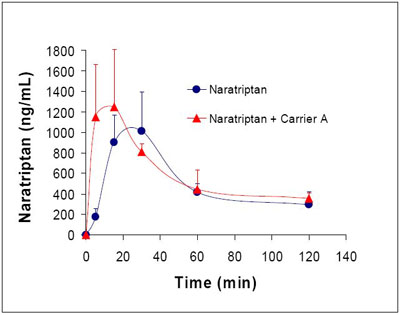
Fig. 4: Pharmacokinetic profile comparing absorption in rates of naratriptan with Eligen Technology (Carrier A) and without
Eligen Technology has been shown to enhance oral delivery of many different therapeutic molecules. This technology works especially well with water-soluble drugs, both positively and negatively charged. Enhanced oral delivery has been demonstrated in the clinic with large drugs (10–25 kilodaltons molecular weight such as unfractionated heparin and growth hormone), medium size biomolecules (3–6 kilodaltons such as peptides like calcitonin and insulin, as well as low-molecular weight heparin) and small molecules (300–1,300 daltons, such as cromolyn and cyanocobalamin).
For drugs with low aqueous solubility Eligen Technology has been less successful but in certain cases it can enhance oral bioavalability.
references:
1. Karsdal M.A. et al. Osteoarthritis Cartilage 2010, 18, 150-9
2. Arbit, E. et al. Thrombosis J. 2006, 10
3. Ding, X. et al. Pharmaceutical Res. 2004, 21, 2196-2206
4. Novick, S.C. et al. J. Clin. Oncol. 2008, 8592.
5. Malkov, D. et al. Curr. Drug Delivery, 2005, 191-7.
6. Mousa, S.A. et al. J. Clin. Pharmacol. 2007, 1508-20.
7. Castelli, M.C. et al. FASEB J. 2010.
8. Castelli, M.C. et al. ASCPT, 2008.
footnote:
Eligen Technology is a registered trademark of Emisphere Technologies
