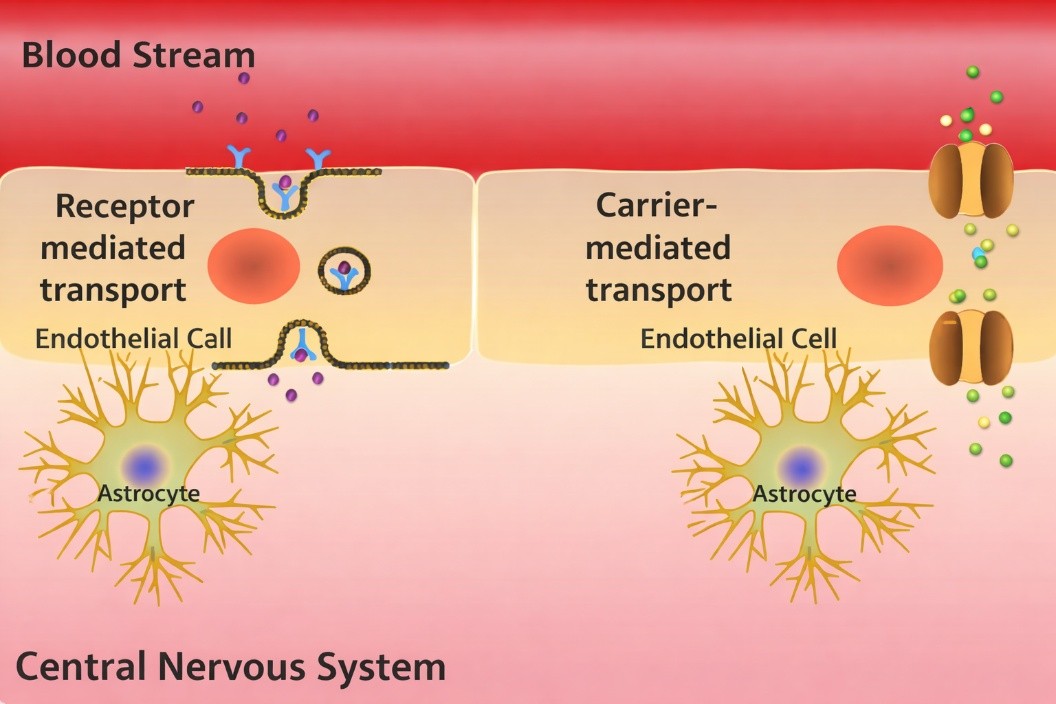
MetP Pharma has published preclinical studies highlighting the differentiated advantages of its proprietary nose-to-brain drug delivery technology (DDT) compared with systemic blood-brain barrier (BBB) transporter approaches such as BrainShuttle or other targeting approaches.
While recent years have seen increased interest in receptor-mediated BBB transport systems, MetP Pharma emphasises that effective brain delivery does not require high systemic exposure or complex molecular shuttles.
Instead, its technology leverages direct neural pathways uniquely accessible via intranasal administration, including the olfactory and trigeminal pathways.
Nose-to-brain (N2B) delivery is designed to achieve high, localised concentration peaks in the brain by leveraging short neural diffusion pathways, enabling a rapid onset of action for highly potent compounds.
This can be decisive in clinical development, where safety, efficacy and CNS exposure determine approval or failure.
When appropriately designed, intranasal neural targeting can achieve higher relative brain exposure and lower systemic plasma levels than intravenous transporter-based systems.
Superior brain exposure without systemic burden
Brain targeting efficiency is commonly assessed by calculating the percentage of injected dose reaching the brain (ID%).
Most approved CNS drugs achieve <1% ID and for peptides, 1-5% ID combined with a brain-to-plasma ratio >1is considered excellent targeting efficiency. Until recently, it was widely believed that only receptor-mediated transcytosis could reliably reach this threshold.
However, MetP Pharma has published previously unreported preclinical datademonstrating that its nasal semaglutide gel achieves the following:
- Brain-to-plasma ratios well above one
- Time- and dose-dependent brain exposure
- ID% values within the highly successful range for peptides.
These findings provide strong support for therapeutically relevant clinical effects of semaglutide using MetP Pharma’s DDT, including appetite suppression and modulation of food reward and addiction diseases.
 Clear differentiation versus transporter-based BBB technologies
Clear differentiation versus transporter-based BBB technologies
Compared with BrainShuttle and other transporter-based approaches, MetP Pharma’s DDT offers several decisive advantages, including the following:
- Non-invasive, patient-friendly delivery
- Unlike IV-based transporter systems, MetP Pharma’s technology enables simple, non-invasive self-administration, making it suitable for chronic, repeat dosing and improved patient adherence
- Reduced systemic exposure
- Direct brain targeting minimises systemic circulation, lowering required doses and reducing off-target uptake and peripheral side effects associated with BBB shuttle approaches
- Lower development complexity and cost
- A formulation-based approach avoids the complexity, cost and immunogenicity risks of engineered antibody or fusion-protein shuttles
- No BBB receptor interference
- Nose-to-brain delivery bypasses BBB receptors entirely, avoiding potential long-term safety concerns linked to chronic receptor engagement.
Nose-to-brain delivery for clinical and commercial success
Despite the rapid growth of the N2B field, misconceptions persist due to exaggerated claims, inconsistent study designs, difficulty in quantifying brain targeting and confusion with local or systemic delivery.
MetP Pharma emphasises that bioavailability comparisons with intravenous dosing are methodologically inappropriate, as the absence of plasma exposure is a feature, not a limitation, of effective neural targeting.
Dr Claudia Mattern, Chief Scientific Officer of MetP Pharma, said: "It is not possible to calculate the bioavailability for the N2B technology as a comparison with intravenous administration would be methodologically incorrect due to the lack of plasma availability."
"Asking for IV bioavailability in a nose-to-brain system is like criticising a scalpel for weighing less than a hammer."
A plug-and-play opportunity for existing portfolios
MetP Pharma’s neural targeting DDT is designed as a plug-and-play enabling technology for existing peptide portfolios.
The market increasingly rewards therapies that offer rapid onset, lower doses, improved tolerability and better patient compliance — all areas where MetP Pharma’s approach delivers clear advantages.
