Pharma and biopharma manufacturers are seeking ways of increasing efficiency and productivity to reduce costs and time to market while at a minimum maintaining current product quality levels. Continuous processing for the production of both small- and large-molecule drugs offers the potential to increase efficiencies and product quality/consistency, while also reducing the manufacturing and environmental footprints and overall costs of operations.
While this potential has not yet been confirmed in many cases, most large and many small pharma/biopharma companies and contract manufacturing organisations (CMOs) have invested in continuous manufacturing equipment and technology to investigate its potential benefits.
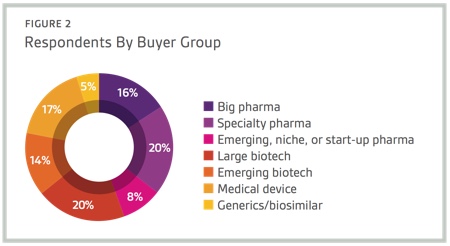
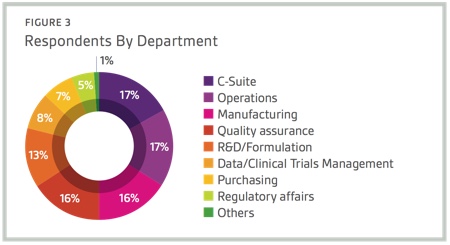
Respondents to Nice Insight’s 2015 Pharmaceutical and Biotechnology Outsourcing Survey of more than 2,300 outsourcing-facing pharma and biotech executives consider technological innovation as important for improving key operations within the pharmaceutical and biopharmaceutical sectors.1 In fact, most survey respondents (96%) have at least some interest in forming outsourcing partnerships with CMOs that adopt state-of-the-art technologies to increase efficiency, safety, quality and traceability. Specifically, 40% indicated they were interested in partnering with a CMO that adopts state-of-the-art technologies.
Huge potential benefits
Continuous processing of small- and large-molecule APIs and formulated drug substances, while requiring very different equipment, offers similar potential benefits. With continuous operation and ongoing inline analysis, process parameters are more closely monitored and maintained at optimal values, leading to more consistent processes and product quality, which in turn can reduce waste generation, product losses and downtime. Because the process runs for long periods of time, smaller reactors can provide large quantities of product. A smaller footprint often equates to lower capital expenditures and can allow for reduced energy, water and raw material consumption, which means lower operating expenses.
Greater automation and reduced human intervention are other important benefits particularly for the production of highly potent APIs and formulated drugs
Greater automation and reduced human intervention are other important benefits particularly for the production of highly potent APIs and formulated drugs. The smaller environmental footprints of continuous processes are also becoming increasingly important as the pharma industry moves towards implementation of green chemistry principles and considers values such as process mass intensity (PMI) and environmental factors (E-factors).
There are also unique benefits for small-molecule production using flow chemistry. Perhaps the most important is the ability to carry out hazardous reactions that would not be possible in a batch process. Because only small quantities of reagents, intermediates and products are present at any given time in a flow reactor, exposure to toxic or energetic substances is minimised, and process parameters such as temperature and pressure can be readily controlled.
For example, Novartis implemented a flow chemistry solution to overcome the hazards posed by Vilsmeier reagents, which are used to convert amides to aryl aldehydes and ketones, but often are irritants and hazardous due to their high thermal energies of decomposition. The technology was applied to the synthesis of the antidiabetic drug vildagliptin.2
Bristol-Myers Squibb also used flow chemistry for an oxidative rearrangement process to minimise the potential for thermal runaway. The process has been implemented at the pilot plant and commercial scale for the production of the investigational liver cancer drug brivanib alaninate.3
GlaxoSmithKline, meanwhile, employed a continuous flow strategy for the synthesis of multi-kg quantities of bromomethyltrifluoro-borate, a key reagent for a Suzuki–Miyaura coupling reaction, due to the highly exothermic nature of the reaction.4
CMOs have also developed continuous processes to overcome hazards associated with batch reactions. For instance, the ring opening of a highly strained (and thus energetic) cyclopropane ring was performed by AMRI in a continuous-flow reactor to increase the safety of a process step in the production of taxadienone on a decagram scale.5 It is interesting to note that safety is the most important metric for respondents of the Nice Insight annual survey when considering a technological innovation that would influence CMO selection.
Perfusion technology, or contiguous biologics production, also offers a particular advantage for biopharma production. In perfusion mode, the media is fed at a constant rate, and spent cells and product are removed continually. As a result, the product is exposed to the culture conditions for a minimum amount of time. Proteins that are highly sensitive and easily degraded under conventional production conditions are therefore often manufactured in a continuous culture process.
Slow adoption rates
Despite the clear advantages and real potential, the adoption of continuous process is proceeding relatively slowly. The main reason for this is the need for further technology development. In the biopharma industry, perfusion technology has been available for many years, but practical continuous solutions for down-stream processing have only recently begun to reach the market. Similarly for small-molecule API production, while some downstream processes, such as distillation and extraction, are well suited to continuous operation, others – particularly solids handling – are more challenging.
Existing infrastructure presents another major roadblock to widespread adoption of continuous processing. Companies are more likely to use existing equipment than to invest in new continuous processing systems, unless there is a distinct advantage in doing so. Thus, at present, continuous/flow chemistry is implemented on a case-by-case basis.
The lack of clear regulatory guidelines also acts as barrier to adoption of continuous manufacturing
The lack of clear regulatory guidelines also acts as barrier to adoption of continuous manufacturing. Harmonised guidelines that are accepted by regulatory bodies around the world are needed to ensure that the monitoring of product outputs from continuous processes is conducted in a consistent manner. Additional approvals of continuous processes and experiences with auditing of continuous process steps are required before this situation can be adequately addressed.
Other issues facing the manufacturers and CMOs considering implementation of continuous processes are resistance to change, the need to integrate activities across different groups and the aversion to investment in new technologies at early process development stages.6 A lack of people with expertise in continuous flow technology is also hindering its adoption. There is both a real dearth of experienced operators and a continuing perception that only people with extensive experience and specific technical know-how can successfully achieve the scale-up and commercialisation of continuous processes. The situation won’t be resolved until university programmes incorporate continuous manufacturing for both small and large molecule drugs into their curricula and more continuous processes are successfully implemented.7
Although the adoption of continuous processing remains limited in both pharma and biopharma, most large companies are investigating the potential benefits.8 In the Nice Insight survey 62% of respondents indicated that they have learned of new technological innovations for the biopharma industry in the past year that would benefit their company. Many CMOs have also recognised the need to have flow/continuous manufacturing capabilities, and leading companies are developing the necessary expertise.
The FDA is also very supportive of continuous manufacturing because it can lead to real increases in product quality consistency. Continuous processes are also generally better characterised and understood, which can only have a positive impact on the final product. The agency has spoken positively about continuous manufacturing since 2004 and has been more vocal in the last year.9 In addition, the proposed 21st Century Cures Act, which seeks to speed up drug development and commercialisation, requires the FDA to support the development and implementation of continuous manufacturing for drugs and biologics.10
For biopharma manufacturing, key advances in upstream continuous production equipment have focused on improving reliability
As well as the development of new continuous crystallisation techniques and equipment, advances are being made in microreactor technology for the continuous manufacture of small-molecule APIs. Collaborative efforts between pharma companies and equipment manufacturers have resulted in microreactor technology suitable for use for pilot and small commercial-scale production, which is important for overcoming scale-up concerns. CMOs including DPx Fine Chemicals and Lonza Custom Manufacturing have leveraged these developments to install commercial-scale continuous production capabilities. Equally important is the expansion of flow chemistry for API synthesis to systems other than those involving liquid reagents and products.
Flow chemistry is also now being applied to reactions that typically were not used in the pharma industry because they are not easy to control or scale up beyond the bench top. Two examples include photochemical and electrochemical reactions.10
For biopharma manufacturing, key advances in upstream continuous production equipment have focused on improving reliability. Upstream technologies that will have an impact include alternating tangential flow systems, continuous centrifuges, acoustic resonance devices and cell settlers. Downstream, real progress has been made with respect to simulated moving bed chromatography, continuous filtration systems, and flow-through absorbers.
One area where significant further development will be crucial is process analytical technology (PAT). In-line analytical monitoring that provides real-time data is crucial for effective implementation of integrated continuous processes. Not only is the information necessary for maintaining optimum operating conditions at each step, it is also necessary to ensure that changes in one step do not affect other integrated downstream processes.
References
1. 2015 Pharmaceutical and Biotechnology Outsourcing Survey, Nice Insight, January 2015
2. L. Pellegatti and J. Sedelmeier, Org. Process Res. Dev, 19 (4), 551–554 (2015)
3. Thomas L. LaPorte et al., Org. Process Res. Dev., 18 (11), 1492–1502 (2014)
4. T. Broom et al., Org. Process Res. Dev. Article ASAP, DOI: 10.1021/op400090a Publication Date (Web): June 10, 2013
5. S. G. Krasutsky et al., Org. Process Res. Dev. 19 (1), 284–289 2015
6. C.A. Challener, 'Potential Benefits Drive Interest in Continuous Manufacturing', Pharm. Tech, May 1, 2014, www.pharmtech.com/bioprocessing-and-sterile-manufacturing
7. C.A. Challener, 'Lack of Expertise Hinders Adoption of Continuous API Synthesis', Pharm. Tech., 39 (7), 30–33 (2015)
8. P. Poechlauer et al., Org. Proc. Res. Dev., 17 (12), 1472–1478 (2013)
9. J.D. Rockoff, 'Drug Making Breaks Away From Its Old Ways', The Wall Street Journal, February 8, 2015, www.wsj.com/articles/drug-making-breaks-away-from-its-old-ways-1423444049, accessed June 2, 2015; J. Wechsler, 'Congress Encourages Modern Drug Manufacturing', Pharmaceutical Technology, May 1, 2015, www.pharmtech.com/congress-encourages-modern-drug-manufacturing, accessed June 2, 2015
10. Energy & Commerce Committee, United States House of Representatives, 'Full Committee Vote on the 21st Century Cures Act', May 19, 2015, http://energycommerce.house.gov/markup/full-committee-vote-21st-century-cures-act, accessed June 2, 2015
