Samsung BioLogics (SBL) has performed N-1 (3,000L) perfusion with the Alternating Tangential Flow (ATF) device to supply a 15,000L commercial production process. The process was carried out in the Plant 3 facility at its manufacturing site in Songdo, S.Korea, reducing production time by up to 30% for the client.
With SBL’s adoption of perfusion technology, it diversifies the company’s portfolio of manufacturing options. Perfusion is gaining broader biopharmaceutical application at small scale with clinical development, but few companies have reported utilisation at large scale for commercial applications to intensify bioprocessing and boost productivity.
Scaling up
The ATF perfusion system at SBL allows for the CDMO to cultivate higher cell culture densities (up to 10 fold) whilst retaining high cell viabilities (>98%) at the seed stage (N-1) to enable inoculation of the production bioreactors (15,000L) at higher cell densities and achieve peak cell densities within shorter culture durations. This allows for improved productivity and reduction in production time, a significant benefit to clients.
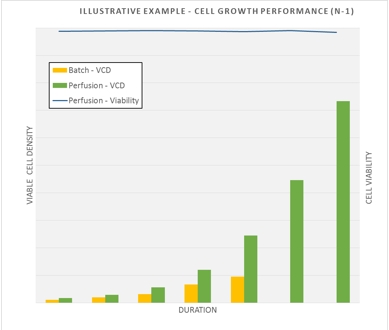
Cell growth performance comparison
During perfusion mode, ATF works continuously by utilising hollow fibre membranes to selectively filter growth medium from mammalian cells with alternating flow to return cells to the bioreactor. A diaphragm pump provides the alternating flow and a low shear environment to minimise cell damage.
The spent growth medium is then discharged, whilst fresh growth medium is continuously added to replace the spent medium at the same rate. The fresh medium allows for continued cell growth and higher cell densities.
A perfusion rate up to 3 vessel volumes per day (VVD) is possible at Samsung BioLogics, utilising three X Cell ATF-10 systems (each 11 sqm) during perfusion mode whilst other bioreactor controls such as volume, temperature, dissolved oxygen, and pH are kept constant.
The perfusion cultivation has also been developed in full automation mode by integrating the ATF system with the bioreactor control logics so that manual intervention is reduced to a minimum during the cultivation duration. Alternate perfusion systems such as Tangential Flow Filtration (TFF) may also be applied with flexible flow path configurations installed.
The ATF system
The ATF system was carefully designed with consultation and support from the vendor. The ATF system (stainless steel filter housing) validation was completed within a six month period including extensive autoclave cycle development and sterility performance testing along with ATF system water and media tests to qualify the operational controls and provide assurance for closed system integrity and sterility.
A lower consumable cost is expected with the fully validated stainless steel ATF filter, although single-use ATF filter units can also be applied in the future.
The ATF system has also been successfully applied for cell culture expansion to meet high cell density targets and production compliant with cGMP operations.
With this successful implementation of large scale N-1 perfusion, clients can leverage another innovative offering to boost their clinical and commercial production capability in addition to the comprehensive array of biopharmaceutical development, manufacturing, and testing services at Samsung BioLogics.
