Although human health relies on smarter technologies, the SCHOTT Smart Container is ushering in a new era of big data analytics for pharmaceutical manufacturers, making it possible to evaluate and optimise common practices and develop levels of automation that have never been possible before. The goal: ensuring that every patient receives the right medicine, safely and in time, because human health matters.
Bringing manufacturing 4.0 to pharma
In innumerable industries, manufacturing is witnessing the rapid digitisation of production lines, creating faster and more efficient methods to measure and monitor production processes. It’s a trend that’s commonly referred to as Industry 4.0, which aims for the comprehensive digitisation of industrial production and the maximum possible degree of self-organised manufacturing as production becomes autonomous.
These developments pave the way for real-time release testing (RTRT), which is the ability to check and ensure the quality of products based on process data. The goal is an immediate, direct release of products based on inline-obtained data.
With new, non-destructive measuring technologies (such as inline residual moisture measurement for lyophilised drugs), real-time measuring systems are on the rise, allowing for further progress toward RTRT. With this, higher yields, improved process control and the increased assurance of product quality could be achieved.
The long-term objective could be the exchange of complex and expensive batch production for continuous manufacturing, wherein materials are continuously in motion, without interruption. To achieve this goal, the full traceability of individual products along the entire manufacturing process is indispensable.
With the Smart Container, SCHOTT adds an additional piece of the puzzle: glass containers that are laser-marked with a unique data matrix code. This enables manufacturers to use big data analytics to record relevant data at various points of the fill-and-finish process and match it to a unique container via a parallel data management system. The result is unprecedented levels of traceability throughout the fill-and-finish process and beyond.
Unique and permanent
Regulations at the national and international level require all pharmaceutical containers to have labels on their secondary packaging. However, current requirements fall short of ensuring full traceability for pharmaceutical products along the entire value chain.
By using a laser to melt a data matrix code onto the container the moment it is manufactured, SCHOTT Smart Containers are permanently labelled. The code is inextricably linked to the container at the earliest possible step in the entire value chain.
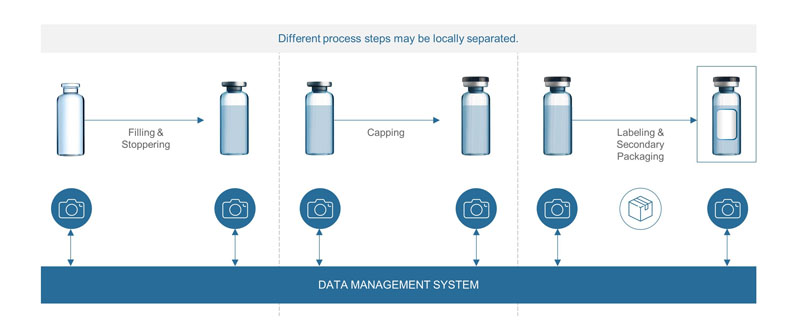
Figure 1: Reducing the risk of mix-ups
This offers a great advantages compared with systems wherein the code is only attached at a later stage. The code is also stable during washing, autoclaving and depyrogenation up to a temperature of 600 °C. It resists abrasion, avoiding the risk of particle contamination compared with solutions that require additional substances for the application of the code.
The data matrix code can be as small as 1 x 1 mm, which equals 14 x 14 dots. Developed according to ISO/IEC 16022, the matrix can be either numeric or alphanumeric, containing 16 or 24 digits. The combinatoric possibilities can reach several sextillion possible individual unique numbers.
For vials, the unique identifier is positioned at the bottom, solving another potential problem: when labels are mounted on the side of the container, manufacturers must install either multiple cameras or equipment that turns the container to ensure the code can be read. With Smart Container, cameras can be installed under the fill-and-finish line itself, ensuring the code is always readable.
Smart containers, intelligent applications
Reducing the risk of mix-ups: With diverting portfolios, the risk of mix-ups during pharmaceutical manufacturing increases. For example, this can occur when filling the same drug in different concentrations at one filling site, or when different steps of the process are performed at different locations (if the vials are kept in storage after filling and before being finally packaged, for example).
The growing risk of potential mix-ups is currently addressed by the use of different capping colours. However, this solution is limited by the number of cap colours available and is prone to error.
With a unique identifier on each container, it is possible to ensure that each container is matched with the right content, cap, label and secondary packaging based on the article data stored in the system. Hence, the content of the container can be identified at any time in the filling process (Figure 1).
Container-based targeted recalls: Because of the significant risks to the future of the business and, most importantly, the health of patients, recalls need to be handled as quickly and effectively as possible. A unique product identifier can greatly contribute here.
The data matrix code on the pharma container can be digitally connected to the secondary packaging, ensuring product traceability at any stage of the supply chain — right down to an individual pharmacy or hospital’s inventory management system.
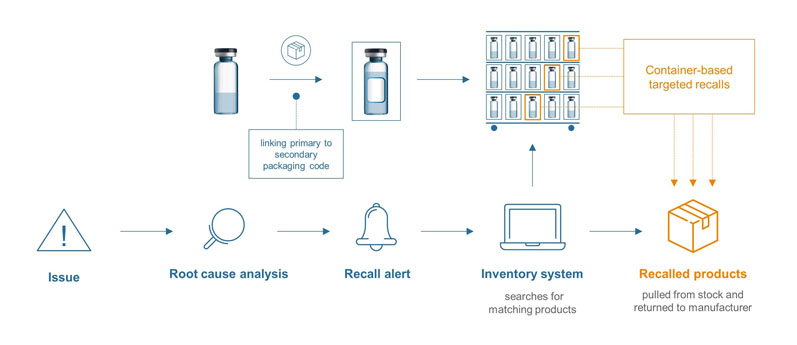
Figure 2: Container-based targeted recalls
In cases when an analysis shows that there is a limited assortment of affected batches, the corresponding products can be tracked via the inventory system for secondary packaging (which is a regulatory obligation) and withdrawn from circulation. The recall can be narrowed down, saving time and reducing costs (Figure 2).
Improved reject management and line clearance: At the end of each batch, it must be ensured that the fill-and-finish line is free from any material related to the previous one (such as vials, stoppers, caps, etc.), so that it is “cleared” for the next batch production.
This procedure is called line clearance, which is done manually and requires a significant amount of time and effort by the operating personnel … while not completely eliminating the risk of remains. Today, IT solutions exist that are able to generate data at both the beginning and the end of the fill-and finish process.
By doing so, stray containers can be identified. So far, however, the data cannot be linked to the individual container and it thus remains unclear at which stage of the process the container was lost. This changes with the SCHOTT Smart Container.
With cameras at different positions on the filling line, each individual container can be tracked throughout the entire fill-and-finish process. Additionally, the reason for rejection can be linked to each container in real-time. Digitally tracked counts can automate line clearance by locating the exact spot of any missing container (Figure 3).
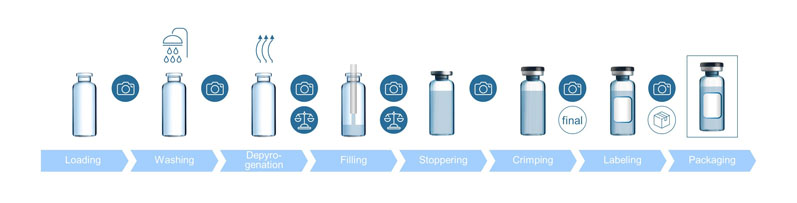
Figure 3: Improved reject management and line clearance
Lyophilisation optimisation: The freeze-drying of pharmaceuticals is a complex and time-consuming process. Defects such as eutectic melting, cake cracking, collapse, lifting, fogging, splashing, puffing or “skin” formation might occur, which lead to unacceptable product.
Therefore, it is crucial to develop a stable, reliable lyophilisation process that is tightly controlled for commercial production. Sensors exist to measure product temperature in real-time, which has the greatest potential impact on product quality.
However, those sensors are only used selectively. With the Smart Container, the exact position of a sensor-tracked vial can be determined, as well as the position of surrounding vials, allowing manufacturers and quality assurance teams to draw conclusions about the lyophilisation process and how to improve it.
The future: supplier data enhances traceability
In the future, the SCHOTT Smart Container concept might even allow pharma manufacturers to receive containers that are pre-encoded with the exact information needed.
This could include relevant production data, information regarding the production site, manufacturing date and time, as well as container-related data such as article number, tubing used, quality level or the results of dimensional and cosmetic inspection.
It is also possible that this data could be aggregated on the label of each tray, which would simplify incoming inspection. Furthermore, if container-related issues during fill-and-finish occur, these could be linked to the received container data.
To summarise, SCHOTT Smart Containers set the stage for Industry 4.0. The highly discrete, robust and durable code solution is inextricably linked to the container and therefore ensures traceability along the entire value chain. Usage on filling lines is simple and cost-efficient, not compromising production speed or increasing risks.
They provide various possibilities to optimise fill-and-finish operations through improved data management on a single-container basis while allowing the user to choose what information to use based on individual needs.

