Some oligonucleotide therapeutics are doubled stranded as opposed to single stranded (see part I). Ans whereas RNA ligase can be used to construct single-stranded products, it also has utility in double-stranded synthesis (innovators should be aware, however, of existing intellectual property.8
To create a double-stranded oligonucleotide, a range of short complementary RNA sequences (natural, unnatural or a mixture) can be assembled. Complementary base-pairing allows the fragments to assemble into a duplex structure, then RNA ligase (or DNA ligase) can be used to join the adjacent fragments together.
Figure 6 illustrates a typical approach for this self-assembly (annealing) and subsequent ligation chemistry. Panels of both RNA and DNA ligases can be screened from bacterial and viral sources, yielding several enzymes with good activity.
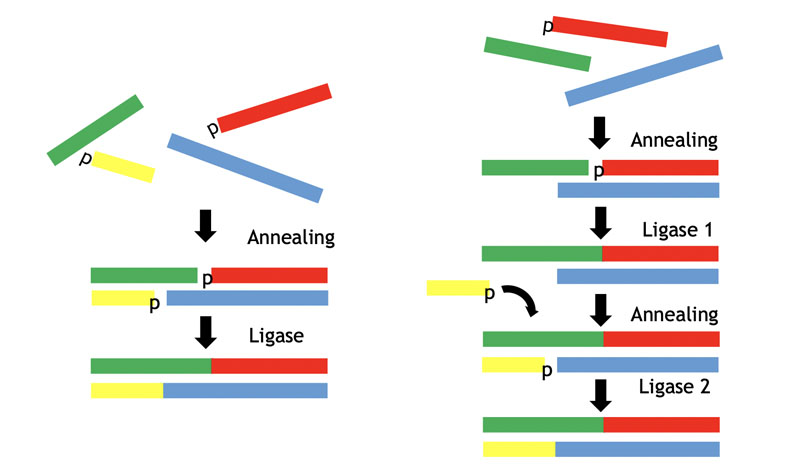
Figure 6: The ligase approach to double-stranded oligos via self-assembly
Almac has shown the importance of enzyme formulation in these types of reactions to minimise background reactions and oligo breakdown. The judicious selection of bacterial hosts and expression systems can prevent unwanted side-reactions by background host enzymes such as nucleases.
To enable low-cost manufacture, enzymes for oligonucleotide synthesis have been formulated as cell-free extracts to avoid expensive purification steps. This methodology should be considered when targeting these reaction classes.
Immobilisation strategies are evolving
It is apparent that oligonucleotide synthesis is a complex and challenging subject. So far, this article has shown how blockmers may be synthesised by single nucleotide addition, how they may be linked to form single strands and how they can also be linked to form double strands.
Numerous additional strategies are evolving, in which the immobilisation of substrates or enzymes is used to increase process performance.9
Immobilised templates have been used to make single-stranded oligos (as shown in Figure 7), whereby Almac has demonstrated proof of principle for oligo assembly using selectAZyme ligases.
These approaches use immobilisation technology in which immobilised substrate templates (blockmers) in various formats guide blockmer assembly prior to subsequent coupling.
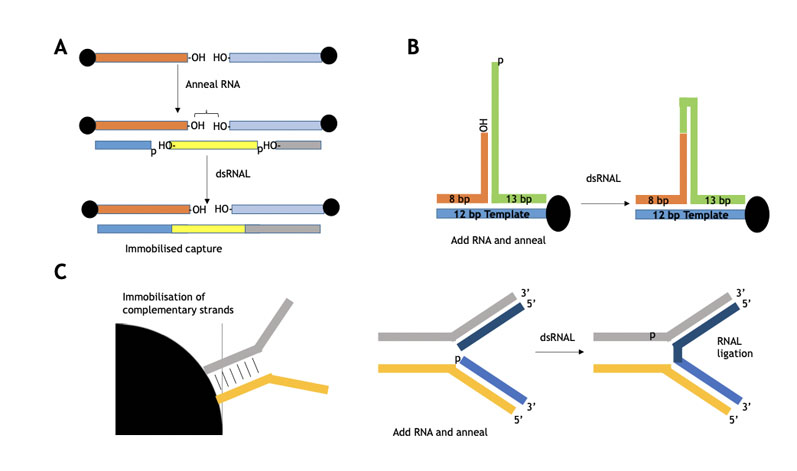
Figure 7: Immobilised blockmers used to assemble two complementary blockers for subsequent selectAZyme ligase mediated ligation
Alternatively, the enzymes themselves may be immobilised. Single nucleotide extension using selectAZyme RNA ligase to generate specific, short, single-strand fragments was outlined in Figure 3.
This same approach is illustrated in Figure 8 using immobilised RNA ligase and alkaline phosphatase for template-free single nucleotide addition. This raises the prospect of a flow-based biocatalytic process with the enzymes immobilised on solid supports and packed in columns as an evolution forward from batch processing.
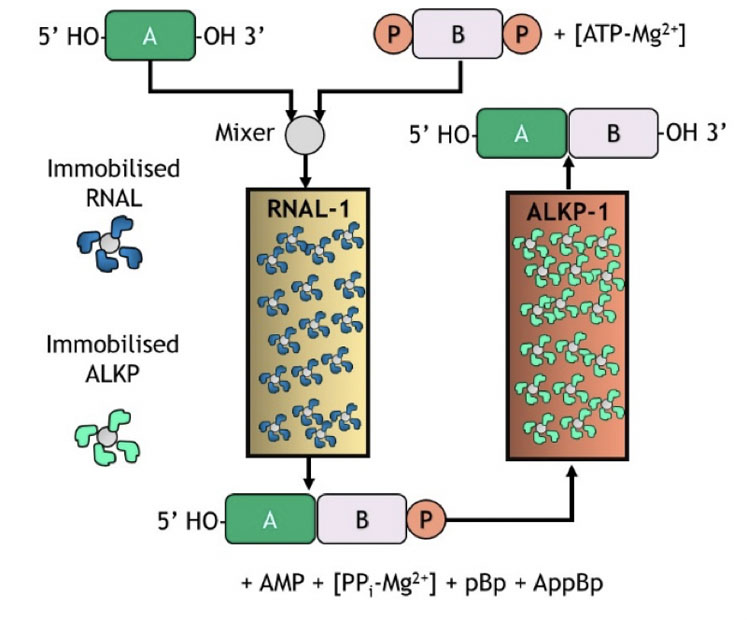
Figure 8: Immobilised selectAZyme ligase and phosphatase for blockmer synthesis
Building block biotransformations
Whatever strategy is taken for oligonucleotide synthesis, it is dependent on having access to the relevant building blocks. Biocatalysis also has an important role in this regard, to mediate regioselective introduction of phosphate groups, for example, or to address stereochemistry requirements.
When sulphur is introduced in place of oxygen in the oligonucleotide backbone, a chiral phosphorous centre is formed. If several sulphur residues are present in the oligonucleotide, then the possibility of multiple diastereomeric products exists.
It is possible to address such chirality issues by resolving mixtures of dimers bonded by a racemic phosphorothioate bond (illustrated in Figure 9).
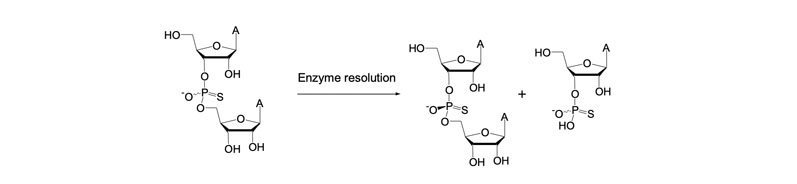
Figure 9: Enzyme-mediated resolution of dimers
Selective hydrolytic bioresolution may be catalysed by phosphodiesterases, phosphotriesterases and phosphoramidases.
In readiness for the next generation of oligonucleotides, wherein a single isomer is needed, Almac has been developing panels of these enzyme classes and taking inspiration from Nature regarding the microbial degradation of organophosphate herbicides to assist and guide enzyme panel design.
These three classes of enzymes have been cloned and screening collections are available to address the need for good stereochemistry control.
When single-stranded products are being elongated enzymatically, it may be necessary to remove a phosphate group to allow the next stage of enzymatic extension.
Alkaline phosphatases are enzymes that cleave an unprotected phosphate under selective and mild conditions (as shown in Figure 3). Almac has developed a panel of 96 diverse phosphatases for this type of reaction.
Modification of the phosphodiester backbone and the ribose ring have been outlined as strategies to improve the therapeutic performance of oligonucleotides.
Another type of modification is in the base when, for example, methylation may be desired. Biocatalysis provides a synthesis route for such modified substrates.
Purine and pyrimidine nucleoside phosphorylases can exchange the natural base for an unnatural base as shown in Figure 10. Almac has assembled panels of diverse purine and pyrimidine nucleoside phosphorylases (PNPs) to assist the construction of nucleoside building blocks when an unnatural base is needed.
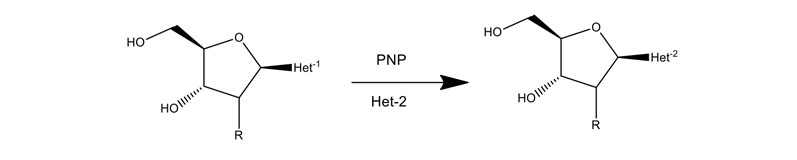
Figure 10: Application of PNPs to make nucleosides
As in oligonucleotide strand synthesis, building block synthesis is greatly assisted by access to various enzyme screening panels; this allows multiple routes and synthetic strategies to rapidly be assessed for a given target.
DNA strands can also be produced enzymatically
Oligonucleotides may be based on DNA rather than RNA, warranting different synthetic approaches. Terminal deoxynucleotidyl transferase (TdT) is a specialised DNA polymerase that does not require a template and adds single nucleotide triphosphates (NTPs) to the 3’ end of an oligonucleotide or DNA molecule.
This is in contrast to the examples above that show how larger fragments may be joined together. If the donor NTP is blocked, for example with a 3’-O-allyl or 3’-aminoxy group, then it can be added to an oligonucleotide by TdT … but indiscriminate further addition of NTPs by the enzyme is prevented by the blocking group.
The product can be unblocked and the cycle repeated so that a DNA chain grows as shown in Figure 11. This pioneering approach may form the basis of future enzymatic oligonucleotide synthesisers and, in time, may also be applicable for unnatural DNA-based oligonucleotides.
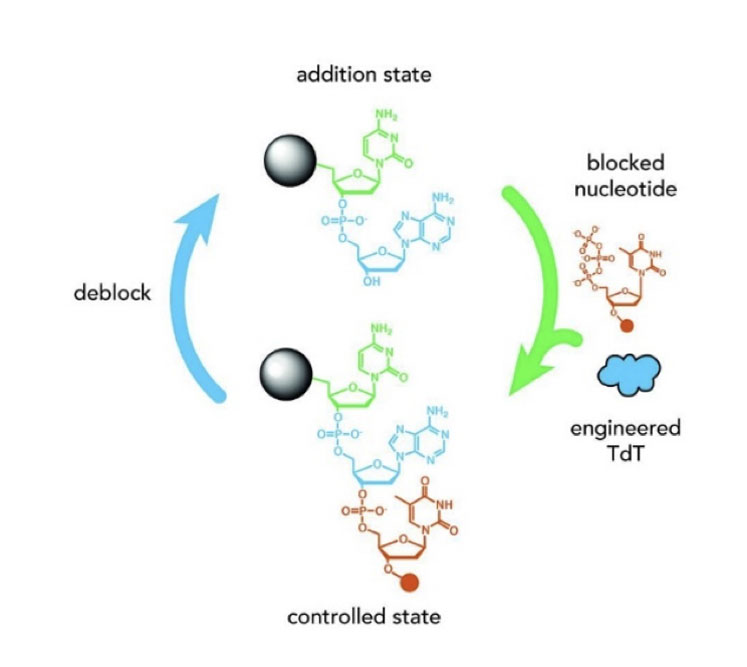
Figure 11: Application of TdT enzymes to assemble DNA
Summary
An increasing demand for oligonucleotides as therapeutics has focused attention on methods for their synthesis. Chemical routes using phosphoramidites have drawbacks relating to the yield and purity of the product and this has stimulated considerable interest in biocatalytic approaches.
Enzymes offer the benefits of sustainability, selectivity and cost reduction in pharmaceutical synthesis. There are multiple synthetic strategies for oligonucleotides (and their building blocks) and these products invariably have considerable structural complexity.
Enzymatic synthesis therefore requires a sophisticated suite of enzyme technologies to effectively tackle both building block and strand construction. Almac has developed a range of biocatalytic products and services to facilitate the challenges posed in biocatalytic oligonucleotide synthesis and we can expect spectacular success stories to emerge in the coming years.
Conclusion
The proven ability of biocatalytic technology to produce hard cost savings for pre-existing processes or to provide economical access to NCEs in the pharmaceutical sector ensures increased year-on-year investment in this area.10
There is a phalanx of oligonucleotides coming through the pipelines of innovators that all need materials for clinical trials. As these products move through the pipeline, larger quantities will be required, putting intense pressure on existing solid-phase capacities.
It is time to think of alternative methods and to reap the benefits of enzyme catalysis in your oligo synthesis. The work showcased here is not only alleviating pressure on capacity, it is unlocking new routes of synthesis and improving the profiles of products that are challenging to purify. Now is the time to unlock the power of enzymes for your oligo project.
References
8. www.chemicalsknowledgehub.com/article/14595/.
9. R. Mullin and T.S. Moody, Chemical & Engineering News 97, 34–35 (2019).
10. T.S. Moody and S. Mix, Specialty Chemicals Magazine, 22–25, (February 2019).
For more information
Jill Caswell, Darren Gray, Daniel F.A.R. Dourado and Steve Taylor, Arran Chemical Company, and Tom Moody, Almac Sciences.

