NICE recently rejected NHS funding of Kymriah for DLBCL despite recognising that the drug has significant clinical benefits. As a result, patients with DLBCL in the UK could now potentially lose out on receiving life-saving treatment, according to GlobalData, a data and analytics company.
Following the EMA approval of Novartis’ Kymriah (tisagenlecleucel) for refractory/relapsed (R/R) diffuse large B-cell lymphoma (DLBCL) in adults last month, 19 September, 2018, NICE rejected NHS funding of Kymriah for DLBCL.
The NHS was preparing to embrace the breakthrough CAR-T cancer treatment and the company offered a confidential discount on the list price of £282,000. Nevertheless the discounted price was still above the range that NICE considers an acceptable use (no more than £50,000 per year) of NHS funds on an end-of-life drug.
NICE based its decision on the lack of data comparing Kymriah to the gold-standard chemotherapy and concluded that Kymriah is not cost-effective for routine NHS funding or use within the Cancer Drugs Fund (CDF).
In contrast, on 5 September, 2018, NICE gave the green light to Kymriah for patients with R/R B-cell acute lymphoblastic leukemia (ALL) up to 25 years old, a much smaller market, within a record-breaking 10 days just after it obtained marketing authorisation from the EMA.
Dr Edit Kovalcsik, Managing Analyst at GlobalData, said: “NICE’s rejection of Kymriah for DLBCL does not come as a complete surprise after a series of dismissals of other immune-oncology drugs for regular NHS funding, including Gilead/Kite’s Yescarta (axicabtagene ciloleucel) in R/R DLBCL and R/R primary mediastinal large B-cell lymphoma (PMBCL)."
“Amid the uncertainty of access to new cancer drugs, on the other hand, the UK is keen to become a global hub in biotherapeutics.”
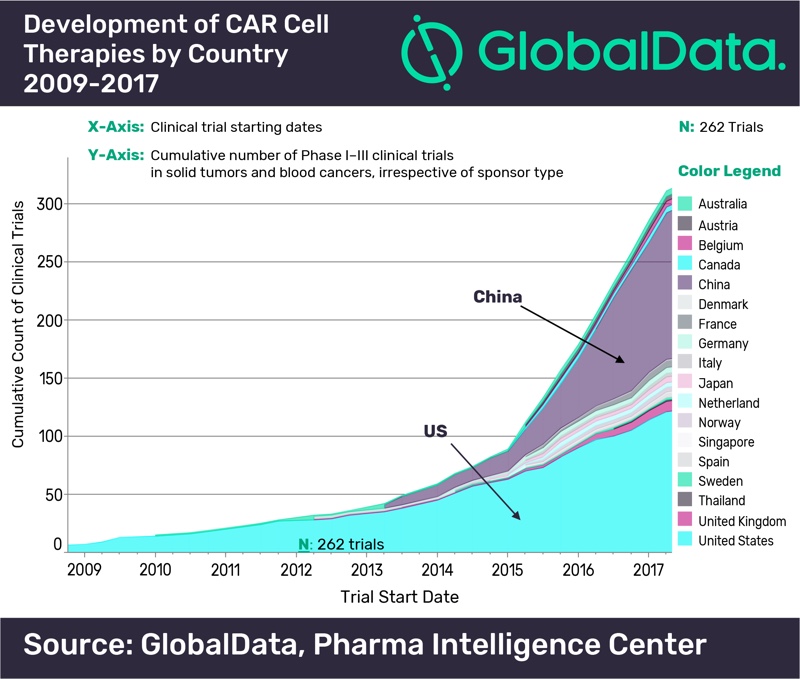
According to GlobalData’s report: 'PharmaFocus: Visual Analysis of Immuno-Oncology Development and Opportunities' the US has historically led the development of CAR cell therapies, but China has recently become the most significant contributor to their development, with 126 clinical trials started prior to Q2 2017 in comparison with 121 in the US. The UK is the third country in terms of development, far behind, with nine clinical trials exploring CAR cell therapies.
To speed up progress in immunotherapy to fight against cancer, UCL, King’s College London, Queen Mary, University of London, and the Francis Crick Insitute teamed up on 17 September, 2018 to establish a virtual ‘London centre’, a collaboration made possible by a £14 million catalyst from Cancer Research UK. As a result, more Londoners will be able to access cancer trials and treatments that can extend life or even have the potential to cure cancer.
Kovalcsik concluded: “Beyond access to clinical trials, the uncertainty of NHS patients to receive the best cancer treatment needs to be addressed. Exploitation of digital technology and artificial intelligence in the analysis of the NHS electronic health records could increase effectiveness of drug development and as a result reduce the cost of breakthrough medicine.”
