New data from Phesi has revealed that more than 100 diseases are now being investigated in the context of GLP-1 usage.
The data analytics company examined 583 recruiting clinical trials, or trials about to start, that involve GLP-1 receptor antagonists.
These include trials directly evaluating GLP-1s as interventions, as well as those exploring comorbidities and modulatory effects.
The data showed that research into GLP-1s has now expanded to encompass more than 100 disease areas.
The analysis leverages real-world data from Phesi’s Trial Accelerator platform and a newly published Digital Patient Profile (DPP) of GLP-1 patients.
The findings reflect growing interest in GLP-1s as a broader modulatory pathway across multiple indications, including cardiovascular disease, polycystic ovary disease, osteoarthritis and multiple cancers.
“Scientific curiosity is increasingly centred on understanding how weight and metabolic factors intersect with other disease areas, including cardiovascular, inflammatory and neurological conditions,” said Dr Gen Li, Founder and President, Phesi.
“The data show a clear trajectory – GLP-1s began in diabetes, moved into obesity and now we’re seeing increasing application across a spectrum of diseases with shared risk profiles."
"The goal for the industry is not just treating obesity, but treating the entire constellation of conditions associated with it."
"GLP-1s are forcing a re-evaluation of how we define, diagnose and treat disease – prevention is emerging as the new blockbuster."
"With the right data, sponsors can target the right patients earlier, saving time, money and lives.”
Phesi’s analysis reveals that GLP-1 use is rapidly growing, with many patients presenting overlapping conditions, such as hypercholesterolaemia and hyperlipidaemia.
This suggests that GLP-1s are increasingly used to target systemic disease clusters rather than single indications.
In some cases, GLP-1s are used adjunctively in non-obesity-related treatments (e.g. osteoarthritis), where initial findings suggest possible synergistic effects.
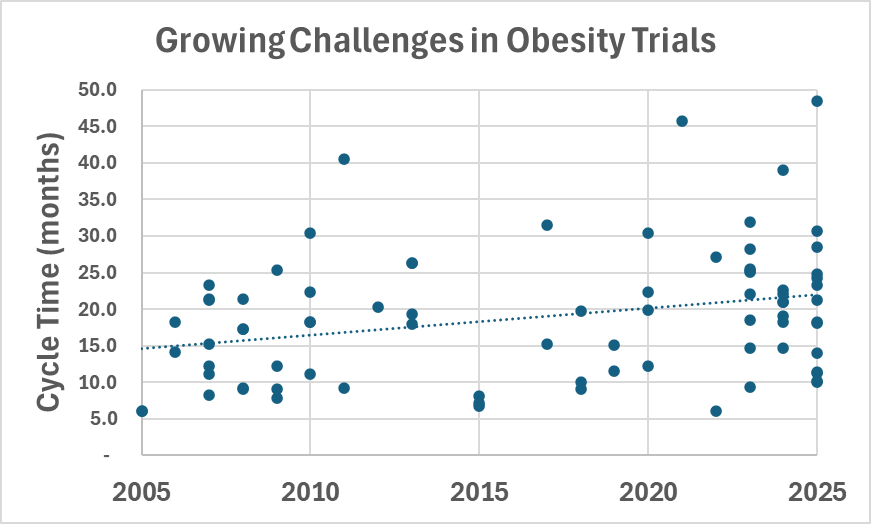 Looking at how this uptick in activity is impacting obesity trials, Phesi finds that cycle times are increasing.
Looking at how this uptick in activity is impacting obesity trials, Phesi finds that cycle times are increasing.
The average duration of obesity trials has grown during the past two decades, with trials that previously took 10-20 months now taking 25-45 months.
The implications of these trends are significant for R&D, clinical trial design and healthcare strategies.
GLP-1s exemplify the shift from disease treatment in isolated departments to a holistic, patient-centred approach, where a single intervention could address multiple conditions.
As conditions such as cardiovascular disease, MASH and diabetes converge, clinical specialities will need to adapt, requiring physicians and trial designers to shift from single-disease expertise to multi-indication decision-making.
Operationally, clinical development organisations need to become smarter and data-driven to ensure portfolios and programmes remain viable against this evolving backdrop.
“GLP-1s have the potential to reshape how we think about disease prevention, not just treatment.
And as GLP-1 usage grows, the volume of real-world data (RWD) is increasing rapidly,” said Jonathan Peachey, Chief Operating Officer, Phesi.
“This may present a challenge to clinical development with competition and recruitment bottlenecks. But it also offers both an opportunity and a solution."
"Sophisticated clinical data analytics combining RWD and disease modelling will unlock insights that optimise clinical operations, reduce costs, drive down patient and investigator site burden and enhance regulatory strategies."
"These insights will also afford sponsors and clinicians a deeper understanding of longitudinal outcomes in treated patients and provide data that can be fed back into analysis."
"This is critical because we are at the early stages of understanding disease convergence and hypotheses are still emerging that will require significant RWD to substantiate.”
Phesi’s DPP is created from analysing 1,896,194 patients about to be treated by GLP-1 inhibitors from 69,652 hospitals and clinics in 81 countries in the past 20 years.
The findings confirm that fasting plasma glucose concentration is correlated with HbA1c, but also find no apparent correlation between BMI and HbA1c, confirming that BMI is not always an accurate measure of health.
A DPP offers sponsors a statistical view of real-world patient attributes, such as demographics, comorbidities and baseline outcomes, to improve trial design, create digital twins and identify top enrolling sites, enabling faster development with higher-quality data.
Phesi’s DPP provides the crucial foundation for sponsors to make faster, smarter decisions in a fast-evolving therapeutic landscape defined by GLP-1 convergence.
