Sulfuric acid (H2SO4) is widely used in the manufacturing processes of various industries. To ensure the quality of the goods these industries produce, it is important to be able to determine accurately the concentration of sulfuric acid at all stages of the process. Anton Paar has the solution — quick and easy concentration determination using the predefined sulfuric acid method for Abbemat refractometers.
How to determine sulfuric acid content quantitatively
For determination of the sulfuric acid concentration, titration is the conventional method. This is a time-consuming and labour-intensive process. Additionally, sulfuric acid’s hygroscopic behaviour can lead to changes in concentration over the time taken to complete titration. The longer the titration process, the greater the deviations in concentration could be. Reduced accuracy of the concentration measurement would be the result.
The quick and easy solution is refractometry. The measuring principle is based on sending a beam of single wavelength light through a measuring prism in contact with the sample. Depending on the difference of the refractive indices between the sulfuric acid solution and prism, the light is partly refracted and reflected, or totally reflected. The critical angle of total reflection is determined by measurement of the reflected light intensity on a CCD array and yields the refractive index.
Refractive index measurements require only a few drops of sample, lowering the cost of wasted sample and its disposal, and increasing the environmental friendliness of the measuring process compared with titration. Because temperature is the most influential factor in refractive index measurements, all Anton Paar refractometers are equipped with a built-in temperature control. This ensures a rapid and even temperature distribution for each sample, compared with a time-consuming and less accurate external water bath used for temperature control in Abbe-type refractometers.
Anton Paar’s automatic refractometers combine sophisticated features like acceptance limits, data recording and communication as well as internal temperature control with an accurate and fast refractive index or concentration determination. The measured values are completely independent of the operator and therefore appropriate for implementation within a standardised measuring process. Anton Paar’s refractometers offer full traceability of all results.
Anton Paar’s method for easy determination of sulfuric acid content in g/100g
Anton Paar has created an easy-to-use method for the determination of sulfuric acid concentrations in g/100g (%mas). The method is based on the correlation between refractive index and concentration of H2SO4 in g/100g aqueous sulfuric acid solution at 20°C. The function in the range from 0g/100g to 100g/100g aqueous sulfuric acid solution is represented by an inverse parabola and therefore passes through a maximum. The reason for that is the emergence of additional binding forces between the molecules (hydrogen-bridge formation). The molecule’s formation requires less space which leads to a volume contraction of the solution.
In the higher concentration range one refractive index is correlated to two different sulfuric acid contents. That is why Anton Paar splits the function into two ranges, from 0g/100g to 84.5g/100g with up to ±0.028g/100g accuracy (see Figure 1) and from 87.0g/100g to 98.0g/100g aqueous sulfuric acid solution with up to ±0.060g/100g accuracy (see Figure 2).
The reproducibility and repeatability (up to 0.3g/100g in the range of 0.0g/100g to 84.5g/100g; up to 0.5g/100g in the range of 87.0g/100g to 98.0g/100g) of this method have been thoroughly tested in Anton Paar’s laboratories.
Within 20 to 30 seconds, the instrument displays the result. On top of this, the calculated mole fraction in mol/100mol (%mol) is filed in each Abbemat refractometer and can be read out easily.
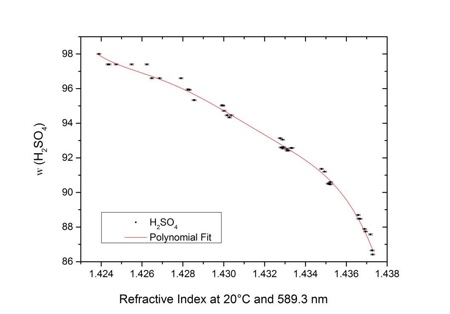
Figure 2: Correlation of refractive index and mass fraction of aqueous sulfuric acid solution in the range of 87.0g/100g to 98.0g/100g aqueous sulfuric acid solution. The refractive index correlates with the content of sulfuric acid with an accuracy of up to ±0.060 g/100g aqueous sulfuric acid solution at 20 °C for the Abbemat refractometers from Anton Paar
Anton Paar offers the appropriate accessories and equipment for safe determination of sulfuric acid content. Micro-flow cells made of PFA and sample wells made of highly corrosion-resistant Hastelloy guarantee full durability for a long product life.
| Table 1: Specifications of the sulfuric acid method for Abbemat refractometers supplied by Anton Paar | ||
| Range [g/100g] | Accuracy [g/100g] | Reproducibility and repeatability [g/100g] |
| 0.0g/100g to 84.5g/100g | up to ±0.028g/100g | up to ±0.3g/100g |
| 87.0g/100g to 98.0g/100g | up to ±0.060g/100g | up to ±0.5 g/100g |
Conclusion
The sulfuric acid content in g/100g (%mas) and mol/100mol (%mol) can easily and quickly be determined with Anton Paar’s automatic Abbemat refractometers. The use of correct sulfuric acid content is important, and Anton Paar has a quick and easy solution to measure it.
For further information please email info.gb@anton-paar.com .

