Rice University scientists have developed a new drug delivery platform that could make it easier to complete a therapeutic regimen and even boost drug efficacy.
 The research, published in Nature Nanotechnology, introduces a peptide hydrogel that functions as a three-dimensional net which controls the rate of release across a range of medication types, including small-molecule drugs and biologics like insulin and antibodies.
The research, published in Nature Nanotechnology, introduces a peptide hydrogel that functions as a three-dimensional net which controls the rate of release across a range of medication types, including small-molecule drugs and biologics like insulin and antibodies.
The system, called self-assembling boronate ester release (SABER), uses reversible chemical bonds between two chemical groups — one located on the peptide and one on the drug molecule — to extend the duration of drug release.
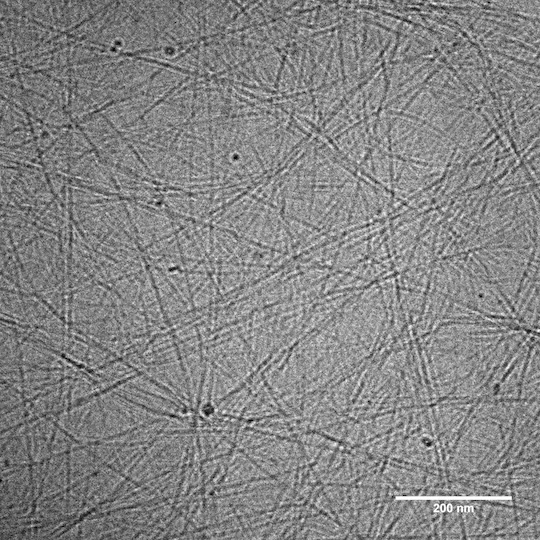 Consequently, instead of passing quickly through the net, the drug gets temporarily “stuck” each time it binds to the peptide, slowing its passage out of the hydrogel.
Consequently, instead of passing quickly through the net, the drug gets temporarily “stuck” each time it binds to the peptide, slowing its passage out of the hydrogel.
The SABER platform could improve treatment adherence and outcomes for existing therapies and open new possibilities for more precise delivery in areas like cancer immunotherapy, where controlling the timing and location of drug release could help reduce harmful side effects.
Brett Pogostin, a Rice doctoral alum who led the development of SABER, said: “I was interested in bridging the two labs and figuring out how I could combine my chemistry and bioengineering interests to do something impactful."
“A major challenge of using hydrogels in drug delivery is that they often rapidly release small drugs,” he said.
“You can imagine you’re a fisherman out at sea and you’re trying to catch minnows, but you’re using a net made for tuna. The minnows swim right through.”
Pogostin hypothesised that dynamic covalent chemistry could be used to make the hydrogel net “sticky.”
By chemically modifying the hydrogel to interact with boronic acid-bearing drugs, he was able to slow release through reversible bonding.
The team demonstrated the platform in two different use cases.
Tuberculosis remains one of the world’s deadliest infectious diseases, not for lack of effective treatments, but because patients in low-resource settings often struggle with access, storage and adherence to long treatment schedules.
SABER not only simplified dosing but also enhanced the efficacy of the drug.
On the biologics side, the researchers turned to insulin, a notoriously difficult-to-dose hormone that requires careful timing.
In scenarios where cold storage and frequent injections are barriers, the benefits to Type 1 diabetes patients of a simpler and longer-acting way to administer insulin could be significant.
As the hydrogel is made of amino acids, it breaks down naturally in the body. After injection, it forms a small, temporary nodule under the skin that gradually dissolves with no toxic byproducts.
The path from initial idea to developed platform was anything but straightforward, requiring broad interdisciplinary coordination.
The team’s research was supported by multiple collaborators and work is underway to make it even more widely applicable, with next-generation applications being explored.
