SmartSkin Technologies has announced the launch of SmartSkin Seal Assurance, a breakthrough solution that gives pharmaceutical manufacturers real-time visibility into seal tightness, leading to Container Closure Integrity across vial and cartridge capping.
SmartSkin will debut the technology at the upcoming ISPE 4.0 in Barcelona, Spain, highlighting how analytics advance seal quality and reliability in pharmaceutical manufacturing.
SmartSkin Seal Assurance provides per-head, real-time feedback on sealing performance by measuring and visualising forces during capping.
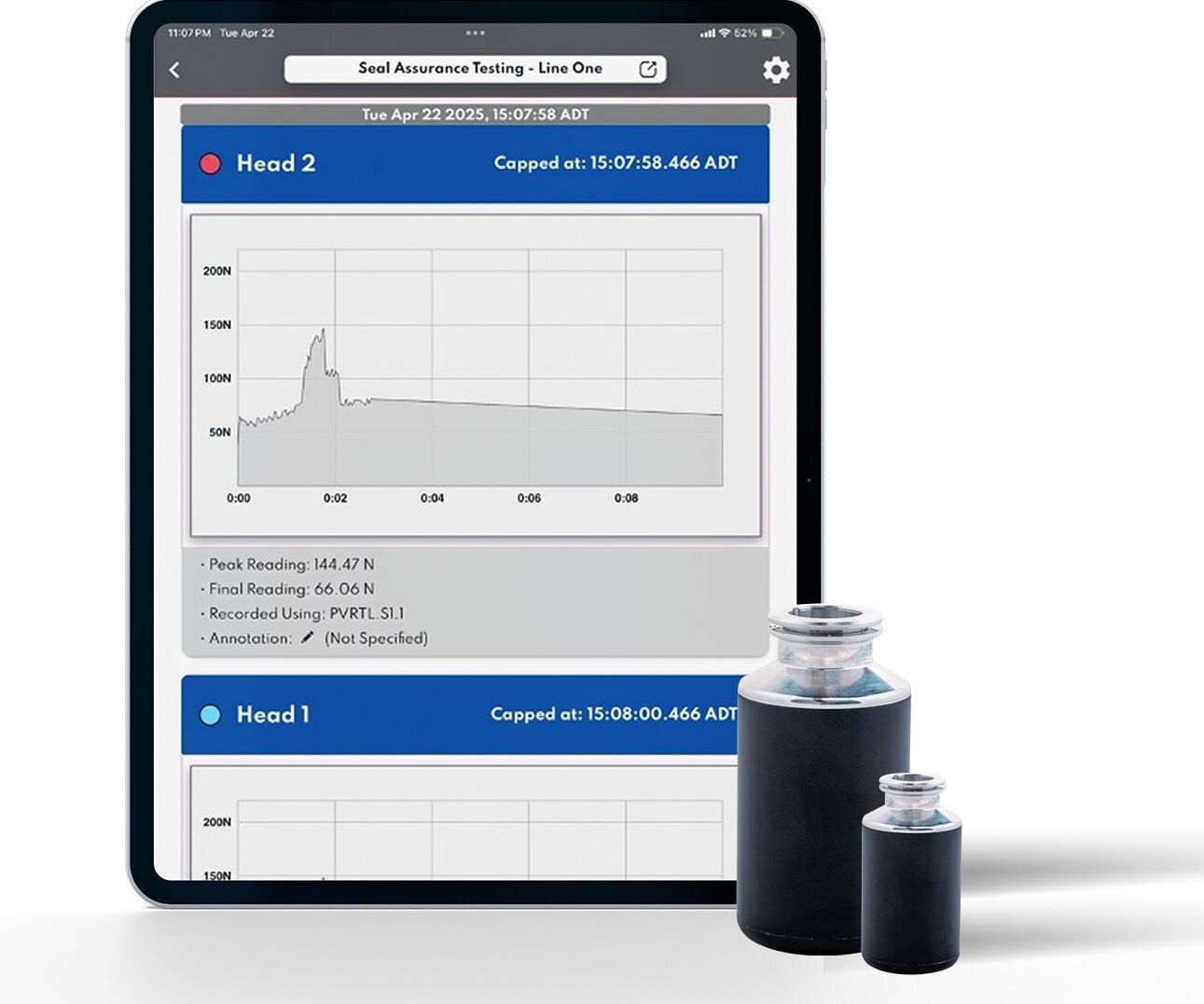 Each event generates a digital signature, creating auditable proof that every capper head is configured and operating within specification.
Each event generates a digital signature, creating auditable proof that every capper head is configured and operating within specification.
This record helps confirm setup quality and consistency.
Compatible with single and multi-head systems, it complements Residual Seal Force (RSF) testing by confirming that sealing conditions measured in RSF are established from the start, closing the loop on Container Closure Integrity assurance.
"Despite the critical importance of seal quality for sterile products, translating the mechanical settings of capping equipment into the physical seal characteristics needed for container-closure performance has not been possible…until now," said Dr Mihaela Simianu, Scientific Advisor at SmartSkin Technologies.
"SmartSkin's Seal Assurance opens the possibilities to proactively build container closure integrity assurance into the aseptic process design, creating new possibilities to enhance equipment and process performance in measurable, controllable and reliable ways."
A recent implementation with one of the world's largest pharma manufacturers demonstrated Seal Assurance's ability to detect head-specific inconsistencies and identify optimal capping parameters, validating its role as a proactive control solution that enhances both quality and efficiency.
Seal Assurance supports applications from factory acceptance testing (FAT/SAT) and line qualification to routine setup verification and troubleshooting.
