Currently, the production and final filling of cell and gene therapeutics is mainly done manually and, in part, on an individual basis for single patients.
It is only possible to achieve the cost reductions needed — while maintaining the same, reproducible quality in the production and use of these therapies — by standardising, digitising and automating manufacturing processes.
Engineering companies work closely with biotech and pharmaceutical companies to develop highly standardised and automated platforms for the production of cell and gene therapeutics at an industrial scale.
These high quality, sensitive products demand flexible machine solutions with high levels of operational reliability and a small footprint.
As the field of advanced therapies matures, there is also a trend towards much greater recognition of the importance of fill and finish in the development and commercialisation of advanced therapies.
The main differences for those products compared with more traditional biologics are the batch sizes and the time needed to process the batch. Batch sizes usually do not exceed a thousand vials for allogeneic cell products and a maximum of 5000 vials for viral vectors.
Cell therapy products in particular need to be processed in a time window of 2–3 hours to prevent affecting the quality of the product.
It is therefore essential that the fill and finish process is done on equipment that’s capable of meeting these criteria, all the while considering process reliability and the safety of both the product and the procedure.
Again, most companies are aware of these requirements and the importance of a reliable and safe fill and finish process; however, it is still the case that some initial discussions only take place when a company is already in later clinical phases or close to commercial production.
The earlier the collaboration with a fill and finish supplier starts, the deeper the understanding of the product and its manufacturing and filling process can be developed and, with that, the appropriate technical solution identified.
Production isolators with individual process sequences
The fabrication of cell and gene therapeutics involves numerous individual process steps. As well as the upstream steps, such as cell selection, enrichment and expansion, there are downstream stages that include purification and final formulation.
This also involves the filling of the product into bags or specific vials that can be cryopreserved. To ensure a closed and safe processing line, fully integrated system solutions that cover the entire production process chain, right through to final filling, are needed.
An industrial solution for a closed production platform to manufacture cell and gene therapeutics is a modular, isolator-based system (Figure 1).
Such a set-up ensures that there is maximum flexibility in terms of process sequence and the ability to integrate external devices such as incubators.
Here, the primary focus is on maintaining what is referred to as scale-out functionality; that is, the multiplication of individual process steps, which makes it possible to simultaneously produce multiple batches (particularly for autologous cell therapies).
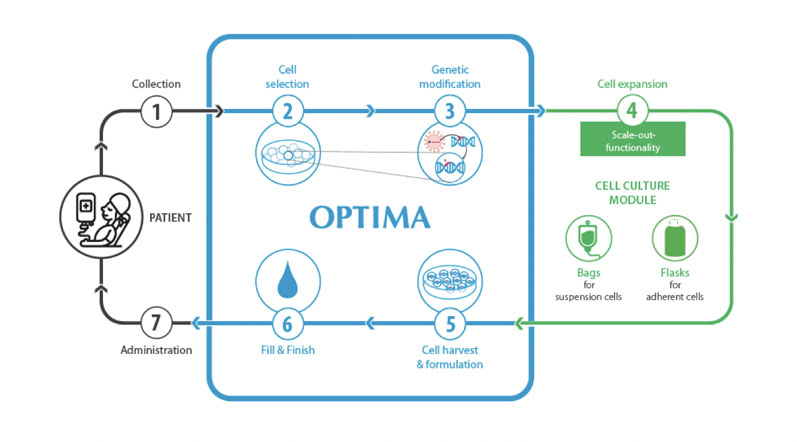
Figure 1: As the operator and product are always separated from each other by the use of isolator technology, all relevant process steps can be mapped and done safely
Such a modular and flexible production platform can be used to produce autologous and small-scale allogeneic therapeutics, suspension cells such as CAR-T cells and adherent cells including mesenchymal stem cells.
All the relevant process steps, from cell selection, cell activation, genetic modification, cell expansion, cell harvesting and formulation to filling into bags, can be mapped and safely performed because the operator and the product always remain separated.
The customer can decide on the level of automation they need as a wide range of variants is available — from manual isolators with an integrated centrifuge to fully automated expansion stages.
As a result, the needs of many different advanced therapy producers — such as clinics, research institutions, pharmaceutical contract manufacturers, start-up companies and large pharmaceutical companies — are met on an equal, personalised basis, and the processes are standardised, safe, reproducible and efficient.
Safe filling with 100% in-process control
Fill and finish machines for cell and gene therapeutics are primarily geared to meet the needs of the customer’s product to be processed. The product is what determines which vial or container will be used.
Here, particular care must be taken to meet the specific processing requirements of vials for cryopreservation. The products are very expensive, so it is extremely important to keep product loss to a minimum and avoid any shear stress, which could damage the product.
Likewise, the selection of the right dosing system is of outmost importance. Additionally, a range of integrated technologies known as “product saving features” ensure high product yield and maximum cost efficiency.
These include a variety of redosing, restoppering and recapping functions, as well as a 100% in-process control.
The use of robot technology allows for a high degree of flexibility and, most often, the filling machine is placed in an isolator to ensure maximum product and process safety by separating the operator and product during the aseptic filling process.
Isolator technology is accompanied by a fast and safe decontamination system using H2O2 for material transfer and decontamination of the whole processing area.
In conclusion, the wide variety of ATMPs requires flexible and reliable machine solutions with a product-specific container infeed and filling technologies to ensure high product and process safety for the fill and finish of these products.
A step closer
Overall, this insight reflects the rapid development and strong market dynamics in the field of cell and gene therapies. Associated with the increasing importance of industrial-scale production and fill and finish, it underlines why special attention should be paid to this market.
With many new technical innovations in the pipeline, we are a step closer to delivering cell and gene therapies to a broader population of patients.
Key points to address in cell and gene therapy production/fill and finish
Production
- Closed production platform using proven isolator technology
- Scale-out functionality with standardised interfaces for external devices (incubators, bioreactors, etc.)
- Modular structure and control with the option to create bespoke process sequences
- High level of process reliability through automation
Fill and Finish
- Closed solutions to fill and close using isolator technology
- High flexibility by implementing robot technology
- High level of process reliability through automation
