With the future of the planet in mind, however, and in response to the European Climate Law — and the need for stronger climate action — the European Commission reviewed the existing 2014 F-gas Regulation.1,2 As a result, in April 2022, a new regulation was proposed to the European Parliament and the Council of the European Union. Building on the success of its predecessor, the F-gas Regulation (EU) 2024/573 was subsequently adopted and has been in force since 11 March 2024.3
Key measures of the new F-gas Regulation include the following objectives: a stricter quota system and new restrictions will be implemented, there will be more comprehensive monitoring and an extension of the phase-down to 2050 (necessary changes from the Kigali Agreement).4
Reducing hydrofluorocarbons (HFCs): The so-called quota system highlights a steeper reduction in the amounts of HFCs that importers and producers can place on the EU market. What was previously referred to as a “gradual phase down” regarding the amount of carbon dioxide (CO2)-equivalent of F-gases (HFCs) is now a “phase-out.” As from 2050, the permissible quantity of HFCs in the EU is zero!

Expanding the quota system: Additional prohibitions on F-gas equipment, products and use of F-gases will apply in the future.
Stricter rules to prevent emissions: The Regulation covers additional equipment and gases, expanding measures to prevent leakage during the transportation, installation, servicing, and disposal of equipment and products.
Facilitating better monitoring: More digitalisation and electronic automation of custom control will allow enhanced enforcement and monitoring in the Member States and combat illegal trade.
Capping EU production of HFCs: Starting in 2025, producers will receive rights equivalent to 60% of their average annual production from 2011 to 2013. This rate will decline to 15% by 2036.
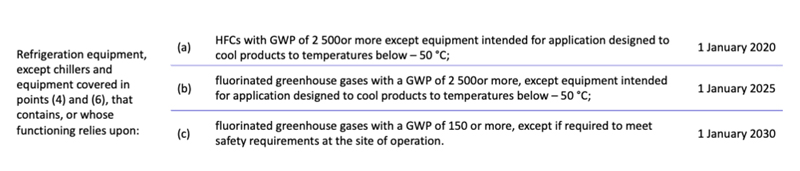
Existing pharmaceutical freeze dryers still use HFC refrigerants that fall under this regulation. In some instances, new systems are even still being planned with them. For the time being, an exception is in place for applications that operate at temperatures of less than –50 °C. However, from 2030 onwards, this exemption will only apply to refrigerants with a global warming potential (GWP) of <150. This applies to new facilities and the maintenance/servicing of extant lines.
The previously mentioned “export bans” will affect all non-EU countries (given that the EU is regarded as a single market). This means that, as of 12 March 2025, refrigeration systems with HFCs that have a GWP greater than 1000 may no longer be exported. Of note, it is irrelevant whether the specific system contains the refrigerant. The decisive factor is which refrigerant(s) they need to function. From 2028, exports may only be made to countries that have ratified (bindingly confirmed) the Kigali Agreement.5 Perhaps unsurprisingly, a license is now required for exports — issued by the European Commission — which requires registration in the F-Gas Portal.
Further to this, certification will also be required for any companies working with natural refrigerants. And even persons with a valid certificate must complete refresher courses by 12 March 2029 at the latest (and every 7 years thereafter).
Current Outlook
There will be a review of the regulation by 2030 to determine whether the current bans are still up to date. Price increases and supply problems in terms of HFCs are to be expected and, of course, the use of natural and low-GWP refrigerants is expected to become the norm. Even so, many low-GWP refrigerants are still considered to be per- and polyfluoroalkyl substances (PFAS) ... and could still be banned because of the EU REACH chemicals regulation.
So, where next? There are many questions to be answered, but Dr Benjamin Ledermann and Thomas Beutler from GEA Lyophil GmbH have been giving this pressing matter much thought. In a presentation at the upcoming GMP-PharmaCongress (8/9 April 2025, Wiesbaden, Germany), for example, they will be discussing the changing situation and advising what to look out for when planning new freeze-drying lines.
They’ll address topics such as associated risks for existing systems, whether old HFC refrigerants can be refilled and for how long current HFC refrigerants and required components will be available on the market. The F-Gas Regulation is a European law, but what influence will it have worldwide?
Available Solutions
Forever shining a spotlight on sustainability, GEA Pharma & Healthcare is committed to helping companies in global markets to design and implement more efficient processes with a lower carbon footprint. Through digitalization and a lifecycle approach, we aim to make planet-friendly practices more accessible and impactful.
For our freeze-drying customers, for example, recent developments include innovative solutions to reduce the environmental impact of the lyophilization process, such as the LYOAIR® cooling system.
Using 100% natural refrigerants and highly efficient compressors, the combination of an air cycle system with a carbon dioxide booster significantly reduces energy consumption compared with traditional systems.
Freeze dryers typically have a high associated energy consumption; so, to counteract this problem, GEA has introduced LYOVAC® ECO Mode, which optimizes the process through dynamic condenser temperature control. It also regulates the mushroom valve, meaning that pharmaceutical manufacturers can unlock significant energy savings without compromising product quality.
Our experts can also talk to you about atmospheric spray freeze drying, our journey towards Annex 1 compliance and the implementation of rapid and energy efficient freeze drying with solid-state radio frequency (RF) energy. Not only does this technology deliver benefits in terms of cost and utility efficiencies, but it also enables continuous mode processing instead of traditional batch handling.
GEA believes in the power of adaptation to shape a better tomorrow and we invite you to join us on this transformative journey. Let's turn challenges into opportunities and set new benchmarks for excellence together.
References
https://climate.ec.europa.eu/eu-action/european-climate-law_en.
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R0517.
https://eur-lex.europa.eu/eli/reg/2024/573/oj.
https://climate.ec.europa.eu/eu-action/fluorinated-greenhouse-gases/eu-rules_en#paragraph_3048.
https://treaties.un.org/Pages/ViewDetails.aspx?src=IND&mtdsg_no=XXVII-2-f&chapter=27.

