These benefits include improved patient compliance and the ability to encapsulate most types of solid (granules, powders) and liquid fill formulations (aqueous- or lipid-based). With the increased use of capsule dosage forms, the pharmaceutical industry has made it a priority to produce the perfect soft gelatin delivery system that exhibits consistent quality and integrity, fulfils the end-use requirements of pharmaceutical and nutraceutical applications, and meets consumer demands as well as stringent industry regulations. However, creating an optimally performing softgel capsule is, in most cases, a complex and highly challenging task owing to the dynamic nature of softgel products and the sophisticated technology needed for their manufacture.
Each critical step in the process, from preparation of the gel mass to drying and ribbon formation, for example, can bring with it a multitude of formulation challenges. The overall manufacture of capsules requires extensive expertise and knowledge of each stage of the softgel manufacturing process and the machinery involved — owing to the extensive versatility of gelatin and the possible complexity of its fill contents. Central to softgel capsules and their challenges, and fundamental to promote a smooth and efficient manufacturing process, is a high quality gelatin with the right characteristics and appropriate functional properties. This article outlines the potential challenges that contract development and manufacturing organisations (CDMOs) face when producing softgel capsules, and discusses why the right gelatin and process are crucial to overcoming these issues.
The perfect gelatin
Its abundant availability and range of unique functional capabilities are why gelatin has been a vital ingredient in a number of pharmaceutical and nutraceutical applications. This includes softgel capsules, of which gelatin has been a main shell excipient for more than 80 years. Indeed, capsule manufacturing would not have been possible without gelatin, the safe and only choice for many decades.
Even now, with an increasing number of alternative ingredients and excipients breaking into the market, such as modified starch and carrageenans, gelatin still offers the best future-proof solution to meet existing capsule challenges in the pharmaceutical and nutraceutical industries. For example, differently from gelatin, modified starch is not classified as clean label, because of the intense processing it undergoes. As well as this, gelatin offers superior mechanical resistance and seal quality because of its lower setting/melting points compared with modified starch and carrageenan alternatives.
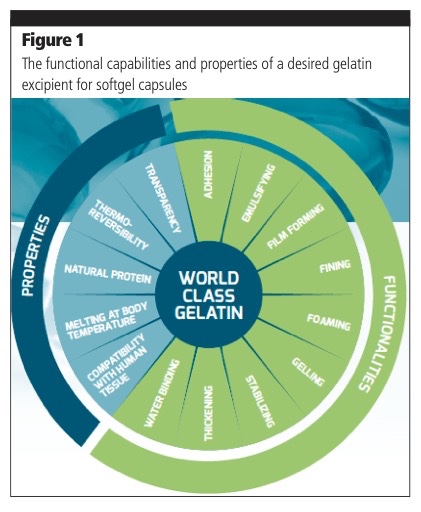
Choosing the right gelatin, with the desired characteristics, is a vital part of creating the perfect softgel capsule and plays a critical role in promoting an efficient and smooth manufacturing process.
Typically, any gelatin offers solubility, ease of use and has good mechanical strength. It also meets growing consumer demands for clean label, natural products and stringent regulatory compliance.
However, to meet the specific needs of the product’s therapeutic action, a specially engineered gelatin exhibiting the desired properties and functionalities is, in most cases, required to avoid softgel defects (Figure 1).
The typical problems that can arise as a result of incorrectly selected gelatin and poor processing conditions have been exposed in tests on lecithin softgel formulations.
For example, results highlight the risk of leakage when softgels were manufactured without using a high wedge temperature, and also showed that gelatin types with a higher molecular weight and high viscosity properties formed seals less readily, resulting in poorly shaped softgels and subsequent leakage. Tests also concluded that, in the conditions of the experiment, alkaline or mixed-process gelatin softgel capsules showed less leakage than gelatins manufactured using acid processes — and optimising the type of gelatin and encapsulation wedge used during softgel manufacture decreased the rate of leakage from 2% to almost 0% (data not shown).
Softgel manufacture
As mentioned previously, a CDMO’s biggest priority is the defect-free and safe production of an optimal softgel delivery system. However, the actual manufacturing process can also prove to be challenging as it involves complex stages including gel mass preparation, ribbon formation and drying, wherein issues can arise at any time.
Importance of gel mass quality
For example, preparing a gel mass that displays consistent formulation performance and minimal foaming is critical to prevent increased production costs and product defects. A deep understanding of gelatin types, their stability and also their foaming capabilities is essential to have full control of the manufacturing process. For instance, it is important for CDMOs to adapt stirring and vacuum conditions accordingly to match the gelatin type to help limit foaming, and thus optimise overall production costs. Selecting the right gelatin with reduced foaming properties will increase the amount of gel mass prepared in the gel melter and will reduce the costs of vacuum treatment (by decreasing deaeration time).
Ribbon forming
Similarly, the ribbon forming stage requires extreme precision and the accurate monitoring and control of the gelatin temperature, ribbon thickness, seam width and fill quantity. At this stage, finding the right viscoelastic behaviour of the gelatin system is essential to create the perfect ribbon for softgel capsules and ultimately ensure production efficiency. For instance, there is a strong relationship between viscosity and film forming, which is strongly influenced by the gelatin’s processing conditions. Therefore, failure to evaluate a gelatin’s film forming and viscosity properties results in poor film forming and quality, and affects the speed at which the ribbon will set.
Drying
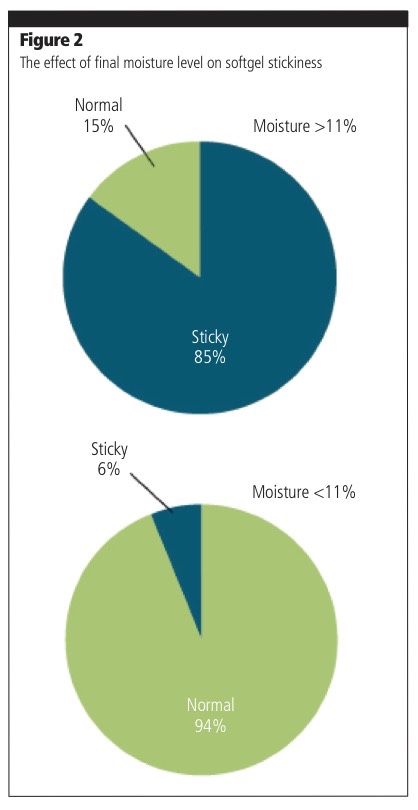
Another critical stage — drying — is an important part of the manufacturing process; it prevents stickiness and helps to preserve the softgels in perfect condition during their shelf-life.
Extensive knowledge of the key parameters at this stage, including drying conditions and kinetics, is essential, as well as a deep understanding of final moisture levels and the need for different drying regimes depending on the plasticiser used (softgels prepared using PEG compared with glycerol or sorbitol).
Research on fish oil softgels has shown that a temperature/relative humidity (RH) combination of 15 °C degrees at 15% RH was the most effective way to reach an optimal lower moisture content in capsules, thus preventing tackiness during the product’s shelf-life.1 However, when final moisture levels are not optimised (greater than 11%), the risk of stickiness is significantly increased (Figure 2).
Yet again though, gelatin type is central to optimising the speed of the process, as gelling proximity, setting temperature and the thermoreversibilty characteristics of gelatin highly influence high speed encapsulation.
For this reason, choosing a gelatin with high solubility, usability and a low viscosity at >50 °C is preferable, as well as optimal mechanical strength and elasticity properties to allow stretching during filling.
Creating the optimal delivery system
However, the challenge doesn’t stop at gelatin types and processing conditions; achieving the optimal API delivery system is also an extremely complex task, mostly because the delivery systems themselves are hugely dynamic and vary widely depending on their fill components and application. Ultimately, the bioavailability of actives in a softgel depends on the dissolution of both its shell and fill, as well as the gastroresistance of the API, and the capsule’s ability to protect the API during storage, only releasing them at the correct time and location in the body once consumed. However, softgels can be sensitive to heat and moisture during their shelf-life, resulting in solute migration, shell inertness and cross-linking during storage, as well as possible softgel instability and inefficient API delivery.
As mentioned, the dynamic nature of softgels is itself a major challenge. For instance, the physical migration of components between shell, fill and external environment, as well as the occurrence of physical and chemical reactions between the shell and fill components, can cause instability, brittleness, shell softness or even loss of shape. Because of such reactions, the capsule can exhibit instability, reduced effectiveness and compromised protection of the API against oxidation, so becoming an inefficient, and possibly unsafe, delivery system. This can be avoided by carefully choosing the gelatin processing techniques and shell formulation.
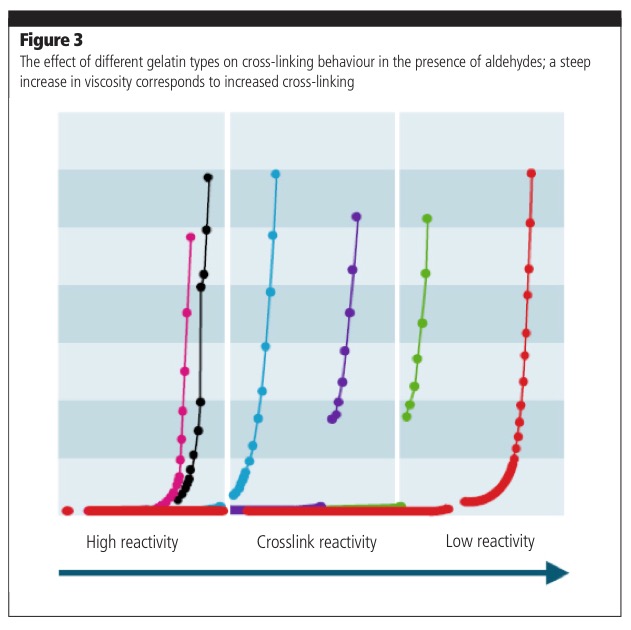
As well as this, extensive cross-linking — the formation of strong chemical linkages between gelatin chains — can cause the shell to become tough and insoluble, affecting softgel stability in storage. Gelatin compositions with the correct weight distribution, a key parameter in cross-linking, have been developed to overcome this problem; therefore, cross-linking and instability can be avoided using a specifically engineered gelatin type (Figure 3).
Conclusion
Evidently, the manufacture of a high-quality and optimally performing softgel capsule that meets end-use requirements, consumer demands and industry regulations is a complex challenge for pharmaceutical and nutraceutical products, requiring a deep understanding of the softgel market, sophisticated technology and the expertise to use it.
Rousselot is a world-leading producer of gelatin with an impressive heritage of 125 years of experience in the production and application of high quality gelatin. It offers CDMOs in the pharmaceutical industry world-class gelatin products and solutions, as well as the expertise and innovation needed for softgel manufacture.
Reference
- M.R.C. Marques, et al., “Liquid-filled Gelatin Capsules,” Pharmacopeial Forum 35(4), 1029–1041 (2009).
This article appeared in the September issue of Manufacturing Chemist.
