There are many data recording options when monitoring critical environments. Ken Appel, manager Regulated Markets, Veriteq, highlights their pros and cons.
There are many competing monitoring technologies and brands of systems purporting to provide for regulatory compliance. The following six such modalities for temperature and humidity monitoring are to be examined in this article:
-
1. Wired systems with UPS power backups
2. Wired systems with UPS power backups and use of Power over Ethernet (PoE)
3. Wireless WiFi
4. Wireless mesh
5. Non-networked/standalone data loggers
6. Chart recorders
Briefly, chart recorders are the oldest technology – paper-based, and powered either by AC or batteries. Standalone non-networked data loggers also use either AC or batteries, and require manual downloading of data at regular intervals. Wired networking technology has been around for decades. While this technology continues to evolve and remains the mainstay of most pharmaceutical operations, wireless has fast become an interesting alternative.
Each method of communicating data has its advantages and disadvantages. When it comes to regulatory-compliant applications involving public health, however, the criteria for using one method over another should be well understood.
Charts 1 and 2 provide an overview of risk factors and cost-of-ownership differences between the continuous monitoring modalities.
Paper-based chart recorders: In the past decade, nearly every leading pharmaceutical company that had relied on paper chart recorders has either replaced them with a network-based system or is about to. Chart recorders can still be found in the marketplace for a few hundred dollars. Most pharmaceutical quality managers consider this technology obsolete due to both the considerable costs of maintaining chart recorder-based monitoring systems, and the obvious risks of handling paper-based records and the lack of, or limited, alarm notification.
It does not go unnoticed that chart recorders rely on humans for regular checks to replace paper, check pens and write deviation reports. In any event, costly staff hours must be devoted to tracking which charts need to be changed when, and which batteries need to be changed, at what intervals. AC-power based chart recorders without batteries offer no ability for continuous data records in the event of power outages.
The possibilities for human error are numerous. At the time of writing, there have been no known instances of regulators rejecting chart recorder-based monitoring systems. However, regulatory agencies encourage the move from manual to automated processes to tighten up quality systems, and make better use of quality resources.
Standalone data loggers: Compared with paper chart recorders, standalone data loggers are not as likely to break. They do, however, incur considerable labour costs for manually downloading data, especially in large plants where hundreds of data loggers are required to ensure environmental standards in both processing and storage areas. These costs are magnified in the current environment where inspection-readiness can be an issue. (Remember the new 15-day period that the FDA requires for responding to observational deficiencies.) Operational costs for complying with regulators requests for information and the interference with normal operations that audits can involve can be considerable. As with chart recorders, the capability to access accurate and complete records throughout the record retention period, as required by FDA 21 CFR Part 11 and EU GMP Annex 11, may be compromised if it takes too long to locate the records or they are incomplete.
There are also multiple human-error sources with standalone data logger based systems. First, staff may neglect to download data before the storage capacity of the instrument is exceeded. Second, battery-powered standalone data logger systems also require ongoing monitoring of batteries, which also creates an opening for lost data, even when so-called battery alerts are in place, because someone has not been there to see it. Third, AC-powered data loggers without batteries may not provide gap-free records in the event of power outages.
If one thinks of technology investments as ways to automate routine tasks to eliminate the costs of labour and potential error, any time there is human intervention standalone data loggers do not generally pass muster. The reliance on human labour to download data, investigate deviations because they were not seen in time, and maintain these files, opens the door to regulatory objections and the staggering costs that ensue with production delays. With the exception of monitoring the contents of a few chambers, standalone recorders put undue risk on companies over other monitoring methods that automate more procedures and reduce reliance on human systems.
Wired networks – with and without PoE capabilities: The pharmaceutical industry has long relied on a wired infrastructure using Ethernet standards for making the connection to transmit and receive data. A hard-wired network allows communications to proceed securely and continuously with few possibilities to intercept or interrupt the flow of data.
Uninterruptable Power Supplies (UPS) ensure that servers are always available for data exchange. However, a potential problem with data continuity of monitoring controlled environments arises with a power outage to the facility. The UPS maintains network uptime but devices connected to the network may be without power, which could mean loss of critical data. Until recently, traditional wired networks lacked a cost-effective alternative to maintain data flow with these critical devices.
Power over Ethernet (PoE), originally implemented for voice over Internet Protocol (VoIP) technology, allows electrical power and data to travel on the same Ethernet cable. Since 2003, companies have been integrating data and power standards on the manufacturing floor with PoE (IEEE 802.af) capable devices.
The advantages of deploying a PoE network are many. For example it:
-
1. Saves the cost of running additional AC power, which usually requires a licensed electrician, aided by the low cost of network switches with built-in PoE power capability;
2. Provides greater flexibility to locate devices around the plant because they can be installed wherever a LAN cable can be run;
3. Increases data communication protection from power outage because the server’s UPS provides backup to PoE connected devices;
4. Uses less energy and is managed from a central location;
5. Protects critical data through the outage period. With PoE, security, maintenance and access can all be managed within an existing IT framework because staff is trained on setting up and maintaining communications networks based on worldwide standards.
Wireless networks – WiFi and Mesh: For many pharmaceutical plants, wireless communications can be a convenient and cost-effective method of connectivity. Ease of installation, reduction in cabling cost and measurements in inaccessible areas are among the major factors driving their adoption.
Unlike the wired 802.3 international standard, several wireless communications protocols have emerged, including the popular wireless version of Ethernet, commonly referred to as WiFi (802.11b and more recently 802.11g). Other network methodologies used for monitoring include a mesh structure based on the Zigbee (802.15.4) protocol.
WiFi is often the wireless system of choice because it uses the same IT infrastructure already in place in an organisation. Wireless mesh (Zigbee) is a network architecture that uses access points or nodes to communicate with one another as well as with the host. It is designed to detect a degraded signal at one access point and reroute it to another nearby access point. Nodes have a low power requirement, which has the expectation of less drain on battery life but at the same time low power inherently means less signal strength than WiFi. The low power requirement of Zigbee networks also means that there needs to be a sufficient number of nodes to maintain continuous data flow.
Whether WiFi or wireless mesh, the greatest downside is the possibilities for network interruptions such as those that occur when lift trucks move throughout a plant or when inventory is re-arranged, equipment is moved out of range or other potential obstacles to transmission and in turn the ability to ensure gap-free records. Fixed obstacles that could block the signal can be overcome using a sufficient number of wireless access devices. Intrusions from a fork lift or storage equipment such as water-based gel packs or office modifications may not be so well anticipated.
WiFi devices connect directly to the company network and uses WiFi access points to transmit data to a central host (server). Mesh devices connect to a gateway that can either host the data or forward to a central server (Figure 1). The range for a wireless device is largely dependent on radio strength, which is also tied to the battery power. Installations using wireless monitoring technology have to accommodate signal range and barriers, which become important factors when continuous data is required. It can mean, for example, that more wireless devices are needed (and greater upfront cost) to ensure network transmission integrity in all situations.
With wireless mesh networks, signals are diverted to maintain data flow but this increases the load on other nodes picking up the signal, having implications for reduced battery life in unpredictable ways. These types of systems need a vigilant source for detecting and alerting for low battery issues well before data is lost.
With wireless systems, signals carrying critical data can also degrade from interfering sources such as other devices communicating in the same 2.4 GHz band (WiFi & Zigbee), and in today’s pharmaceutical plants this especially includes security cameras, microwave ovens and Bluetooth devices. Wireless mesh is a proprietary network that needs to be integrated with the different standards of the existing infrastructure—involving IT hours, which potentially affect costs of ownership.
battery life
The batteries used in wireless devices are specified with ‘up to’ so many months or years of life. The ‘up to’ condition is often stated for ideal or laboratory conditions. This is because there are no typical operating conditions where power consumption can be calculated. Devices draw down battery power with each transmission, which includes the frequency of measurement updates established by the user, events such as alarms or communication problems brought on by many circumstances including a blocked access device.
Battery drain is even less predictable in a mesh infrastructure. For example, when the signal between a temperature device and node is blocked another nearby node picks up the signal for transmission. It now adds the new transmissions to those from other measurement devices it was already communicating with. The extent to which a wireless network requires battery replacements reintroduces human error potentials into what are assumed to be relatively error-proof automated systems.
Most connectivity methods have low risk of losing data when the time between real-time updates for data and alarms is long. For example, the initial requirements of a monitoring system may not have needed frequent data updates, but at some point someone may want to know if a chamber door was left open or other behaviour that led up to a temperature excursion. In these instances, a faster sample rate would be needed, requiring more transmissions and thus making more demands on the battery.
Reduced battery life is well and good if SOPs can anticipate the need, staff have the time for required maintenance without fail, and the expense of more frequent service (labour hours, replacements) is not burdensome. Some devices provide low battery indicators and alarms. However, the question is: What happens to data if batteries are not replaced in time? Moreover, it is highly probable that data will be lost in systems that use the same batteries to power both the wireless radio and data memory electronics.
Battery life therefore has great impact on the ability to ensure compliant gap-free records and minimising impacts of human error on quality assurance systems.
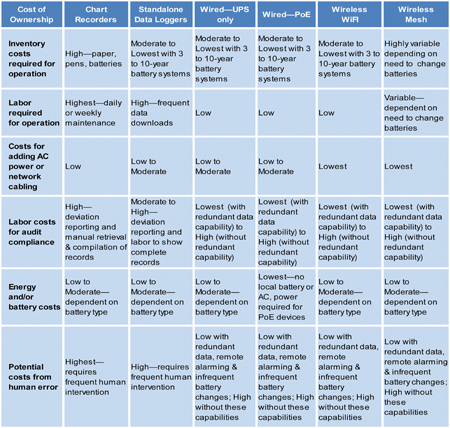
Chart 2: Cost-of-ownership differences between the continuous monitoring modalities
Data redundancy: Whether deploying a system of standalone devices, or a wired or wireless network for monitoring critical environments, the need to have a continuous record of data and events is the same. There will be times when the facility experiences network and power interruptions, among other unexpected disruptions. Assuming the need for a continuous record of quality, the monitoring system should be capable of filling in a database when temperature, relative humidity, pressure and other data cannot be communicated in real-time. This is nearly impossible and impractical with standalone monitoring instruments, making them obsolete technology.
protecting data
In networked systems, recording data independently at the point of measurement is one key factor in protecting data. A system capable of identifying the time period of a communication interruption and bringing in data and events to fill the gap is essential to ensuring complete and accessible records. This assumes measurement devices have calibrated time-base clocks (specified with an accuracy over a temperature range) to ensure correct time/data recording. The capability to backfill data after power or network interruptions ensures a continuous record of data and events. Completeness of records also assumes there is an audit trail to capture all system events.
Documented evidence of data and events during an outage will reduce quality management’s involvement in having to review excursions and investigate deviations. Gap-free records save time during and after an audit, reduce unnecessary staff involvement and limit disruptions to production and shipments.
Data security: There are two aspects of security that regulatory compliance (21 CFR Part 11) requires: protecting data from unauthorised access, and preventing alteration to data. Secure data begins at the measurement device and ends at a designated collection point, usually a network server. Secure access refers to specific levels of permission given to authorised users and other protocols for ensuring authenticity.
Devices communicate using protocols or common rules for data format and can be either open (public) or proprietary. An open protocol means just that – anyone who knows the rules can potentially access the files. A secure monitoring system ensures that the measurement device has a secure protocol in addition to other authentication and confidentiality features. This is a major factor in how and why communicating over wire is inherently more secure. In wired systems devices are accessible only within the building. A wired network can be compromised only by a person who has already gained access to the facility.
On the other hand, wireless communication is inherently less secure and requires additional measures to maintain protection. Wireless devices are also manufactured with security features built in but may not be upgradable to the changing standards or security requirements of an organisation. A capital investment made now may need replacement in two years’ time. Non-standard proprietary wireless networks require IT training and offer increased risk due to IT staff turnovers.
Whether you use standalone monitoring instruments or wired or wireless connectivity for temperature and humidity measurement devices, it is important to understand the limitations of each methodology. For the most part, the significant labour costs involved in standalone monitoring devices combined with the multiple ways in which such systems insert human error potential make them less than desirable compared with more automated network-based monitoring technology.
Wireless communication is flexible to install and provides advantages of monitoring environments that either have limited access to running cable or where refrigerators, freezers or other monitored storage units are moved on a frequent basis. Wired networks have the advantage of speed, security and data redundancy. Generally speaking, if your goal is to reduce the risk of data loss, then wired systems are the best course. The lower upfront cost of wireless can disappear quickly if you have to write deviation reports from missing data, experience product loss or regulatory missteps. The good news is that connectivity technologies can be mixed – wired and wireless – solving the physical installation challenges that many facilities pose.
sources
www.veriteq.com/chart-recorders
