Xellia Pharmaceuticals, a specialty pharmaceutical company focusing on providing important anti-infective treatments against serious and often life-threatening infections, has reported key business updates and financial results for the year ending 31 December 2019.
Business updates
In 2019 the company reports that it expanded its portfolio, partnerships and manufacturing network, while growing revenue and profitability.
Momentum and strong market positions were secured in the US for key products and VANCO READYTM – the first launch from Xellia’s pipeline of formulation improvements.
Xellia entered into a long-term partnership with Civica Rx for several important sterile injectables; and a collaboration with eTon Pharmaceutical, Inc. for the co-promotion of Biorphen, a ready-to-use phenylephrine hydrochloride formulation, post year end.
Xellia also completed substantial upgrades at its Cleveland, Ohio site, transforming the facility into a modern and world-class production hub and enabling the successful cGMP inspection and March 2020 US Food and Drug Administration approval to commence commercial manufacturing of drug products at the site.
The decision was made to divest Xellia’s Raleigh, North Carolina facility to Sagent Pharmaceuticals. This will allow future attention and investments to be focused on Xellia’s facilities in Cleveland and Copenhagen as its two main sterile injectable production hubs.
Financial results
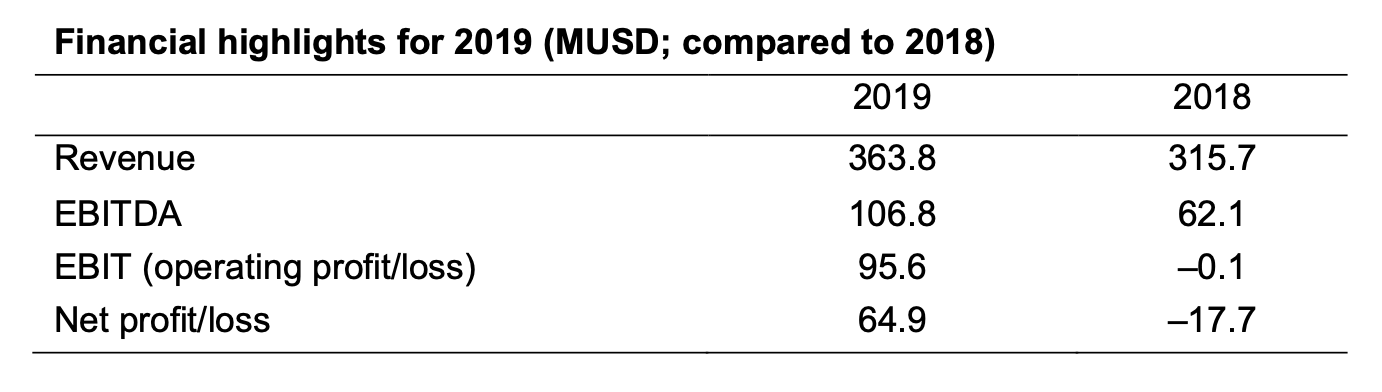
The 2019 financial performance showed significant improvements in both revenue and profitability and were in line with expectations for the year.
Revenue increased by 15% to 363.8 MUSD following a strong performance both in the global industrial business and the newly launched US institutional business.
Profitability increased by 72% with EBITDA of 106.8 MUSD, and the net result for the year was 64.9 MUSD, a substantial increase over the previous year.
Carl-Åke Carlsson, Xellia’s CEO said: “2019 was a very successful year for Xellia, with record financial results and many notable achievements. During the year, we continued to see strong demand for our portfolio across all geographic markets, and we maintained our focus on the reliable supply of anti-infectives to our customer base, as well as pursuing new partnerships and collaborations.
As we move forward in 2020, maintaining our robust supply of vital anti-infective medicines for life-threatening infections has never been more important. In the face of significantly increased demand and supply chain disruption due to the coronavirus pandemic, we will continue to leverage our global network to reinforce our own supply and help to meet any potential global drug shortages of critical anti-infectives.
We remain committed to supporting the market and our customers, and we will work to ensure that patients in need receive their treatments.”
View the corporate report here.
