Modular flow technology for the continuous production of chemicals is becoming the solution of choice when conventional batch reactors fail to deliver the required quality of product for reasons such as difficult-to-control heat transfer, the accumulation of high energy intermediates, mixing issues, etc.
Globally, significant efforts have been made to optimise the manufacturing flexibility and robustness of processes used to produce chemicals in a continuous way, including the application of multiphase flow reactors, gas/liquid mixers and structured catalysts to improve the design and function of flow equipment. Yet, despite these scientific developments, a major challenge for industry is the established application of flow technology to commercially relevant examples.
In response to the ever-changing regulatory landscape of active pharmaceutical ingredient (API) production and market demand, Almac has implemented a continuous flow chemistry platform called flow assisted synthesis technology (FAST), which focuses on four key areas (see Figure 1):
- high pressure hydrogenation
- high energy chemistry
- oxidation
- photochemistry
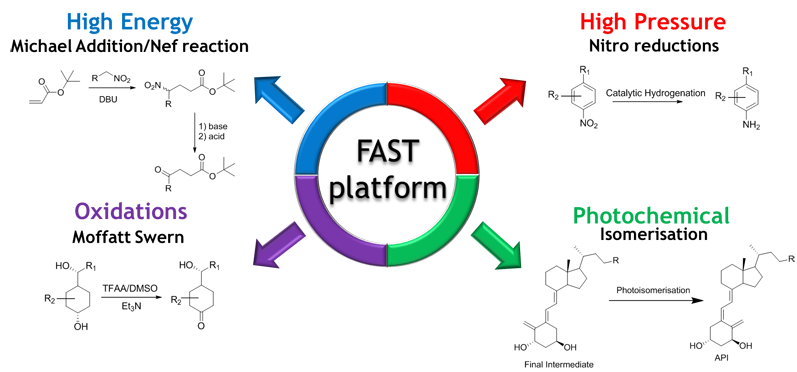
Figure 1: The FAST platform
A driving force behind the need for the adoption of continuous manufacturing as technical innovation is to access reactions that are more difficult to handle in batch mode because of safety and operability issues. Continuous production is of particular interest to companies looking to conduct low-temperature and high-pressure reactions at scale.
Continuous flow offers a variety of advantages compared with traditional batch processing. There are five strategic imperatives to implement flow chemistry, as follows:
Speed: continuous flow reactions are typically faster because of improved mixing and better heat transfer; the smaller-scale architectures offer higher surface area to volume ratios when compared with traditional batch vessels. Reaction rates are also increased by the ability to access temperatures and pressures that are inaccessible with batch-based systems.
Safety: continuous flow is inherently safer; the small volumes reacting at any one time with reactive intermediates/hazardous reagents in situ significantly reduces risk levels.
Challenging projects: reactions that are difficult to scale-up in batch, such as high pressure, high energy, oxidation or photochemical procedures, can readily be performed with continuous flow. This offers Almac’s customers novel and innovative ways to implement new synthetic strategies.
Product quality: tight control of stoichiometry improves overall quality as product is pumped away from the reactants as it’s formed. This results in fewer by-product impurities. Inline monitoring can also be used to identify a change in product quality and allow for diversion to waste, eliminating failed batches. The ability to perform a controlled quench results in a simple work-up, typically with a reduced number of handling steps.
Smaller footprint: continuous flow equipment is much smaller than typical batch vessels; plus, it’s possible to tailor the flow rig to suit the specific process and benefit from higher unit productivity/space-time yields.
Practical application
Almac’s flow chemistry team has implemented a four-stage project workflow to ensure the successful delivery of projects for our clients in acceptable timelines and at a competitive price (see Figure 2). Defined workflows ensure the development of robust, safe and scalable processes for multi-kilogram production.
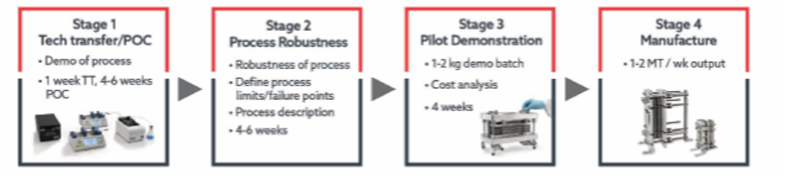
Figure 2: The project workflow
Proof of concept: stage one involves a confirmation of reaction validity step under continuous flow conditions, including an assessment of the solubility of the starting materials, reagents and products, as well as confirmation of product formation. Various parameters will be investigated, such as stoichiometry, flow rates, temperature, etc.
Process robustness: stage two is a process robustness test to define safe operating parameters as the process is further developed for scale-up. This stage also includes confirmation of flowability, hazard assessment and preparation of a process description (PD).
Pilot demonstration: with the PD prepared, a kilogram-scale demo batch will be prepared using intermediate equipment (from pilot to production). This generates material for customer assessment and critical information for further scale-up.
Production: stage four involves building a suitable flow rig, full safety testing of the process and the delivery of the required amount of product according to agreed specifications.
At the end of each stage, a go/no go decision is made in agreement with the customer. As with any new or emerging technology, there is no “one size fits all” approach to flow chemistry; it’s seen as a complementary tool that can expand Almac’s service offerings within the industry and help us to access chemistries that were previously beyond the reach of our current capabilities.
Almac offers continuous flow as a solution to customers when batch-based processes cannot feasibly meet their expectations, such as when hazardous by-products, reactive intermediates or selectivity issues and scale/temperature limits are involved, or if controlled addition is required. The identification of opportunities to apply flow solutions to current processes is also critical to the success of this new technology.

