The objective of risk assessment, especially in drug production, is to proactively identify potential hazards, their likelihood of occurrence and the magnitude of harm they could cause to workers.
By understanding these risks, manufacturers can implement appropriate control measures to prevent or minimise their impact.
According to the law, employers are responsible for evaluating and managing the risks associated with exposure to harmful ingredients.
Between 2017 to 2022, for example, Directive 2004/37/EC — as part of the OSH Framework Directive 89/391/EEC — has enacted four amendments to introduce stricter thresholds for various carcinogens and expanded its applicability to include reprotoxic substances as well as carcinogens and mutagens.
Furthermore, adapting to technical progress and implementing engineered solutions, as opposed to personal protective equipment (PPE), is another established principle of the Framework Directive.
Assessments should be done periodically and the relevant data must be provided to the appropriate authorities on request. Efforts must be made to prevent workers being exposed to harm and to employ closed technological equipment systems.
Key considerations for an effective containment strategy
When it comes to implementing an effective containment strategy, several crucial factors must be considered. The first step is to categorise the product within the appropriate occupational exposure band (OEB).
This allows for a clear understanding of the risks associated with handling the product and establishes the necessary precautions that need to be taken (Figure 1).
Determining the occupational exposure limit (OEL) is essential when setting a safe handling threshold. This limit indicates the maximum concentration of the substance that a worker can be exposed to in a specific time without experiencing adverse effects.
The quantity of the product also plays a significant role when it comes to defining the containment strategy. Handling large quantities may require additional safety measures and specialised equipment.
Understanding the airborne potential of the products is another vital consideration. Certain substances can vary depending on their state — whether still or in motion and/or have a high tendency to disperse — making containment more challenging. In such cases, the strategy might include the use of extraction systems, isolators and sealed containers to prevent inhalation risks.
Factors such as temperature, physical properties (liquids, powders, vapours) and relative humidity (RH) must also be evaluated. Certain substances may react differently under specific conditions, making them more volatile. Adjusting such levels in the workspace can help to maintain the stability of the product and reduce any potential hazards.
Additionally, product characteristics are another key aspect of defining the containment strategy. Some substances may cause skin irritations or allergic reactions upon contact. Identifying these sensitivities is also essential to ensure that employees are adequately protected.
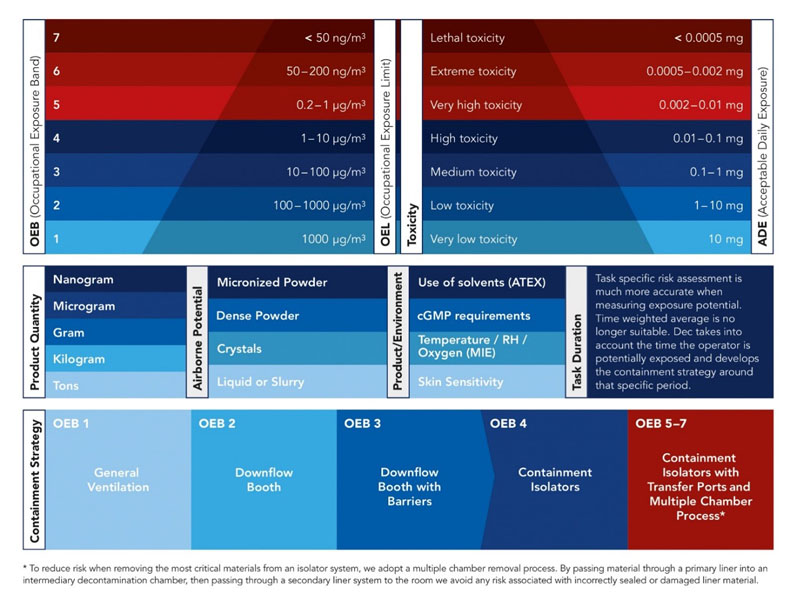
Figure 1: Key considerations when planning process containment
Choosing the right solution
Whether you are dealing with the transfer of dusty products or highly potent ingredients, it is equally crucial to find and use the appropriate containment solution.
The improper handling of ingredients can pose significant contamination risks, compromising both product quality and worker safety. In chemical and pharmaceutical manufacturing operations, one operation that’s particularly susceptible to containment breaches is material transfer.
Areas such as connections between process stages/equipment can pose a significant risk regarding the release of hazardous materials into the surrounding environment or product contamination.
Common transfer solutions include airlocks, rapid transfer port technology (RTP) — to enable the airtight and swift transfer of materials in, out and between different containment systems — and split valves.
Each solution must be evaluated for its suitability based on factors such as product properties and the quantities being transferred. For instance, split valves are not suitable for use with chemical sensitisers, particularly when they are highly micronised, as it is extremely challenging to keep the valve surface free from product contamination (Figure 2).

Figure 2: Powder residue on a split valve
Another approach involves direct transfer with a pneumatic conveyor. However, it’s worth noting that standard vacuum conveyors might present constraints in terms of cleaning, maintenance and the replacement of filters.
Improving efficiency and safety in solids transfer
To maximise containment, safety and productivity, the ultimate answer lies in integrating production equipment using a PTS Powder Transfer System (Figure 3).
This technology has been specifically engineered to handle highly potent and sterile products. Thanks to its exceptional filtration capabilities, it can be cleaned and sterilised in place. Its innovative filter concept enables dense-phase powder transfer under full vacuum.

Figure 3: The PTS Powder Transfer System offers flawless end-to-end process integration
The powder is conveyed at a very low velocity (1–3 m/s) and high concentration (>100 kg powder/kg air), resulting in minimal electrostatic charging, no segregation or particle damage.
Mitigating exposure risks: process containment
Depending on the level of exposure risks involved, various containment strategies are available to handle products safely. When weighing and dispensing less hazardous powders (OEB 2–3), a downflow booth offering airflow control, extraction and the filtration of released particles may be an appropriate solution to prevent particulate matter from entering the operator’s breathing zone and surrounding area (Figure 4).
Working with highly potent active pharmaceutical ingredients (HPAPIs) calls for stringent control measures to achieve the required containment performance target (CPT) and protect workers from potential exposure risks.

Figure 4: Weighing and dispensing excipients in a downflow booth
These ingredients require specialised handling techniques, specifically designed equipment and designated facilities. Implementing a containment strategy, such as isolator or glovebox technology (Figure 5), provides a dedicated area in which these substances can be safely contained, mixed, milled, dispensed and eventually transferred into intermediate or final packaging.
Operated in negative or positive pressure, depending on the process, isolators will protect workers and ensure the purity and sterility of the ingredients.

Figure 5: A wet processing isolator with integrated small-scale glass reactors
Ensuring safe handling, GMP compliance and effectiveness
The following case demonstrates a containment strategy that effectively uses a variety of process systems to achieve maximum containment throughout the entire process (Figure 6).
A new two-stage isolation and handling concept has been recently designed and implemented for a project in a thyroid hormone production facility. This construct ensures closed product handling and achieves a low OEL of <400 ng/m3.
The isolator consists of a conditioning chamber receiving trays from an adjacent process room, which is connected to the tray offloading and milling chamber.
Tray discharge, milling and homogenisation: The milling and blending stage involves collecting the product in a discharge hopper, which is then fed into a cone mill. After milling, the product is transferred to the homogenisation process wherein samples from 2–10 g are collected.
The PTS Batchmixer operates without rotating tools, allowing for gentle homogenising that prevents particle damage. The blender is mounted directly above the pack-off isolator chamber from where it discharges the product into a 50 L feeder hopper.
Pack-off process: The pack-off isolator is a system that consists of two chambers. The upper chamber has an integrated weigh scale that can be moved to the side for direct discharge into the lower chamber.
In the upper chamber, the APIs are filled into bottles and stored on a rack before being transferred to the transfer chamber. The lower chamber allows for the direct discharge of APIs into bags using an Ezi-Dock containment valve system.
The transfer chamber is used to transfer empty containers into the isolator before starting the batch and to transfer filled containers out of the chamber using a RTP continuous liner system to ensure high containment.
Second-phase reactor charging, filter-dryer discharge: The second phase of the process involves charging a reactor with product from previously filled Ezi-Dock bags and filter-dryer discharge.
After filtration, crystallisation, washing and drying, material is transferred to the filter-dryer discharge isolator before being milled and transferred back to the blending process.
In this second phase, the PTS Batchmixer is set to deliver the product in trays to be reintroduced into the isolator conditioning chamber for equilibration purposes (humidification).
The product is then homogenised in the blender and samples are collected. Finally, the product is accurately dosed into 1 kg containers. The PTS Powder Transfer System is used for reactor charging, preventing explosion risks, ensuring effective transfer and dosing both dry and wet powders.
The integrated programmable logic controller (PLC) system oversees both the isolators and the entire process system to operate the plant, which has been designed for clean-in-place operations.

Figure 6: High containment multiprocess operations
In conclusion, developing a comprehensive containment strategy involves a multifaceted approach. Categorising the product, determining occupational exposure limits, considering volumes and handling requirements (airborne potential), and evaluating product characteristics, are all vital elements when it comes to ensuring a safe working environment.
Despite significant advancements in technology, choosing and designing the most suitable concept to meet specific requirements remains challenging.
A thorough understanding of the products, as well as the strengths and shortcomings of each technology, is necessary for a final assessment. It is wise to seek advice from an expert who can evaluate every parameter and suggest the optimal solution for each particular situation.
The Dec Group provides a wide range of process containment solutions to cater to diverse needs.
Whether it’s to handle and process bulk solids, conduct small and/or large molecule research and production, engage in nuclear applications or comprehensive operations encompassing the entire process from receiving materials at the warehouse to delivering the final product, our dedicated teams are happy to help you make the right choice.
