One of the most delicate phases in pharmaceutical production processes is autoclave sterilisation. However, the very concept of sterility is challenging. Arguably, to say that an object or environment is sterile doesn't mean the total absence of contamination, only that it contains the lowest concentration of possible contaminants. Consequently, such a condition needs to be addressed.
Sterility assurance level, or SAL for short, is used to express the probability of bacterial survival. It defines an area as sterile where 1 out of 1,000,000 units is contaminated (the bacterial count equals 10-6).
Sterilisation used to be defined as a process in which all possible life forms, including spores, were completely destroyed by passing an object through an autoclave that would allow the absence of germ proliferation. In time, it became evident that passage through an autoclave doesn't ensure sterility per se: a cycle in an autoclave is a process that must be considered in its entirety, from the preparation of the materials to their storage. None of these phases can be underestimated for a successful sterilisation cycle.
Autoclave: How it works
The autoclave consists of a steel container equipped with a door that can be hermetically sealed from the outside. Through special ductwork, the water vapour coming from a boiler is pumped into the autoclave. The air gradually leaves the equipment until only the pressurised steam remains inside.
The pressure inside the autoclave can reach anything between 6.9 x10-3 kPa (0.7 atm), which corresponds to a water vapour temperature of just over 115°C. Let's not forget that all forms of bacteria, moulds, and yeasts perish after only a few minutes of exposure to 100°C and that the most resistant spores (i.e. those of the tetanus bacillus) crumble at 115°C in 15 seconds. Good practice dictates that sterilisation is continued for 20 minutes to guarantee that all pathogenic and non-pathological forms of any kind are dead and therefore the material inside the autoclave is perfectly sterile.
When operating an autoclave, the following conditions are required to combat bacteria and viruses that form spores effectively:
- The sterilising agent reaches all surfaces of the object to be sterilised. Some materials have a smooth, well-exposed structure, while others are much more articulated and therefore present challenging management
- The steam is in direct contact with the sterilised material. This step involves particular care during the loading phase of the autoclave
- A vacuum is created to move all the air initially present in the autoclave and then replace it with steam
- A well-designed control scheme for steam evacuation and cooling is implemented so that the load does not deteriorate
Process efficiency
The efficiency of the sterilisation process depends on two factors: steam and pressure. Together, they ensure that enough heat is transferred into the organisms to kill them.
A series of negative pressure pulses are used to remove all possible air pockets, while steam penetration is maximised by applying a succession of positive pulses.
The relationship between temperature and pressure is generally the following:
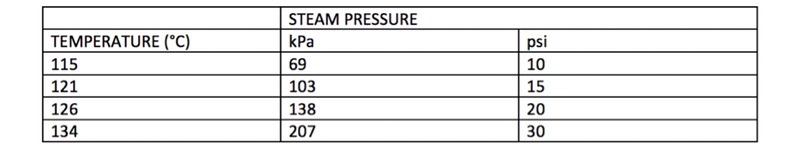
The specifications of the autoclave add another element to factor. Managers should consider both the type of materials and relative pressure loops of the item subject to sterilisation. The table below outlines the different criteria for consideration:
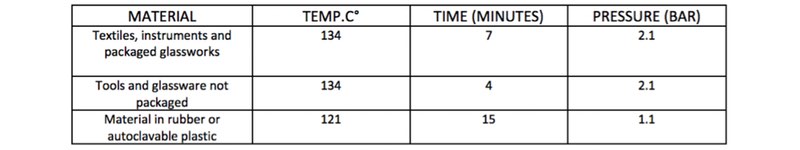
AM Instruments has developed a clear yet essential framework to understand the importance of the phases before and after the sterilisation cycle in an autoclave.
In fact, what has been said regarding the main characteristics of the machine is negated if the risks connected to the preparation of the material to be sterilised are not considered from the time of loading up to the final storage.
Materials in the spotlight
Let's start with the preparation of the material. The microbial charge on an object (bioburden) can be mild, medium or severe. For this reason, it must be effectively removed through decontamination and washing, both of which are essential preventive actions of the sterilisation process.
The subsequent packaging phase has specific purposes, such as:
- Allow the removal of air and, therefore, the penetration and contact of the sterilising agents with the surface of the object to be treated
- Reduce the risk of contamination of the sterilised material when the sterile package is open
- Preserve the sterility of the treated material until it's used
- Be free of toxicity
- Be practical, convenient and economical
On the other hand, "good" packaging depends on several factors:
- Material used
- Packaging methods
- Structure of the object to be packaged
- Storage
Medical paper is the most commonly used for this application, but is also the most at-risk type of material. Medical paper requires a high level of operation and handling with possible damage to the equipment. Plus, the coverage is irregular (a single layer covers some parts, two over others) affecting the passage of steam. Also, it's often difficult to remove. A high risk of fibre release combined with the possibility of tears and damage adds to the risks associated with the use of medical paper. Finally, a critical factor to consider in a production cycle is time; the operational time for packaging is high.

Autoclave topper before and after wrapping in medical paper
An effective and safe packaging method is one that meets specific requirements, such as:
- Designed to offer total security and resistance
- Customisable and easy to use
- Not influenced by autoclave sterilisation processes
- Resistant to tears and perforations
- Easy to apply and remove
- Minimal handling
- No taping if possible
- Low particle release
- Excellent microbiological barrier
- Uniform penetration of steam through the surface
With the above in mind, Tyvek material packaging systems provide a solution. The range is designed to make the preventive preparation operation not only safe for the autoclave cycles of the materials but also to minimise the operating times required by the cycle.
Unlike medical paper, the material in Tyvek generates very few airborne particles when opened or manipulated. This low-lint release property minimises the risk of introducing particulate matter into a clean environment.
A further advantage of using Tyvek material for sterilisable steam bags is that, unlike medical paper, Tyvek maintains its dimensional stability and high-quality visual appearance after steam sterilisation. Even in the most severe conditions in highly contaminated environments, Tyvek has proven to be highly resistant to the penetration of bacterial spores and other contaminating microorganisms.
Particle and bacteriological tests show that it performs better than other commercially available porous packaging materials, including medical paper.
Autoclave loading
Once the packaging has been carried out, autoclave loading represents an equally important step for the success of the sterilisation cycle. It must be performed in such a way that the steam can circulate freely and penetrate each package. Make sure the process follows the checklist below:
- The autoclave load must be evenly distributed and must not touch the internal walls
- The items to be sterilised must be arranged so that each surface is exposed to the sterilising agent for the expected temperature and time
- Special tools, such as containers and pipes, must be arranged with the opening facing downwards to prevent condensation and the formation of air bubbles
Upon completion of the sterilisation cycle, it's necessary to follow other procedures that ensure sterility is maintained: the cycle should be repeated in case of damaged packages or contact with wet surfaces, for example.
Pharmaclean by AM Instruments, the line and production facility launched by the Italian company to minimise the risk of microbiological and particulate contamination, meets the regulatory requirements, with Tyvek material packaging and sterilisation systems. The range, available in standard and custom-made formats, allows the operator minimum manipulation in total safety. The systems are located in A/C grade cleanrooms.

Autoclave topper before and after wrapping in Pharmaclean by AM instruments
With Pharmaclean, microbiological checks are carried out in accordance with Annex 1, and particle checks are performed in accordance with ISO 14644.
The stringent regulatory requirements, combined with the production rhythms of the pharmaceutical industry, make collaboration with suppliers increasingly important, to create optimal solutions that safeguard the product and do not slow down production cycles.
N.B. This article is featured in the July 2019 issue of Cleanroom Technology. The latest digital edition is available online.
