Human blood is a vital resource. It plays a crucial role in transfusions and as a component of specific medicines. Although red at first glance, approximately 55% of human blood consists of a whitish-yellow liquid.
This is plasma, notes Markus Heinz, Product Manager, Vials, at Syntegon Technology, the fluid in which white/red blood cells and platelets move through the body.
What’s essential for the human body also turns out to be advantageous from a pharmaceutical standpoint: blood plasma can be used to produce life-saving drugs.
Owing to its wide range of components, which include important proteins such as albumin, which is used for sepsis and burns, as well as immunoglobulins, these antibodies are primarily used to treat innate defects as well as diseases such as COVID-19.
Products with potential
The market for blood plasma derivatives is growing and is expected to reach a value of $53 billion in 2024. This high demand is being driven by advances in medical technology. More and more compounds can now be produced recombinantly — by genetic engineering — and no longer require donated plasma.
Technologies for fractionation (splitting plasma into its components) have also evolved. Continuously operating centrifuges, for example, and commercial-scale chromatography support more efficient production processes.
This in turn results in higher yields of valuable proteins such as albumin, immunoglobulins and coagulation factors, paving the way for more versatile therapeutic options.
Regardless of recent medical advances, blood coagulation disorders, rare genetic defects and immune diseases are on the rise worldwide, increasing demand for plasma derivatives.

Type 1 diabetes, rheumatoid arthritis and multiple sclerosis have spread rapidly since the late 1980s, especially in Western countries. The US is the central market for blood plasma; whereas roughly 8 million litres are collected in Europe every year, the US accounted for 65% or 38 million litres of global demand in 2020.
From raw material to medicine
Before plasma can be transformed into safe and effective medicines, the raw material must go through several stages of processing. Manufacturers normally receive donated raw plasma in frozen form.
It is then thawed, fractionated, purified, formulated, filled or freeze-dried, inspected and, in some cases, pasteurised. Because of the complex nature of production and the limited availability of human plasma, pharmaceutical producers must ensure precise and reliable processes.
Plasma loss is not only costly, it also means wasted therapeutic opportunities. In addition to high yields, flexible processes that deliver safe, high-quality products are paramount.
Customised thawing and fractionation
The frozen plasma that pharmaceutical manufacturers process is shipped in large bags or bottles containing donor material from several sources. Before fractionation, the raw plasma must be thawed gradually to avoid harming the temperature-sensitive proteins.
This is done in specialised systems with mixing tools that exert low shear forces on the material and gradually raise the temperature. After thawing, the raw material is ready for fractionation; that is, split into components such as albumin, immunoglobulins and fibrinogens, which are among the coagulation factors.
The fractionated plasma then undergoes several filtration steps that separate the target molecules according to size, electrical charge and chemical affinity.
This highly complex step requires equipment that offers efficient and stable large-scale fractionation. All-in-one solutions from a single source form the basis of a seamlessly integrated, fully automated system with continuous monitoring of critical parameters.
Each extracted fraction must be stored and purified separately. Impurities and viruses are degraded chemically or using heat while the concentration of each future active ingredient is increased.
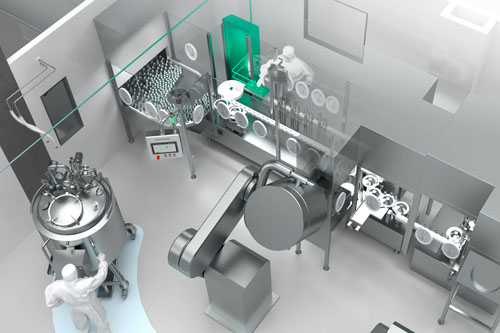
Various excipients (including stabilisers) are added to the fractions during formulation. The stabilisers ensure that the fractions remain effective throughout the subsequent process.
A robust infrastructure for pure media supply complements these steps and provides a reliable source of pure steam and purified or highly purified water.
Gentle and precise dosing
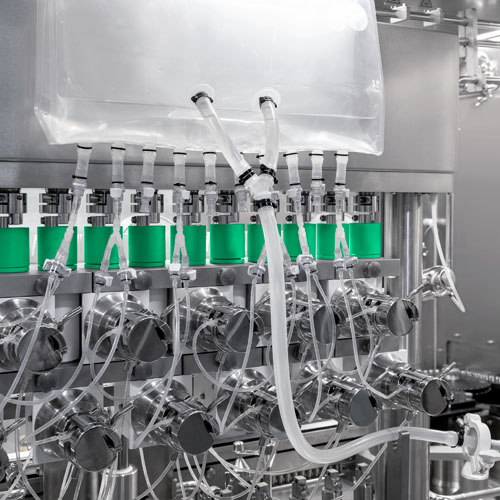
Plasma derivatives are mainly filled in glass vials of varying volumes, typically ranging from 2–500 mL, which are used for liquid and freeze-dried products alike. The demand for flexible filling systems that can accommodate different vial sizes and deliver precise filling volumes is high.
Modern technologies rely on multistage filling and weighing processes to achieve high filling accuracy … even with fast changeovers.
Peristaltic pumps, which are particularly suitable for shear-sensitive proteins such as coagulation factors, are first used to fill 95% of the product. The remaining 5% is filled in a second step — with a smaller filling needle and within more narrow tolerance limits.
This main and subsequent dosing make it possible to determine the optimal filling weight with 100% in-process control (IPC) and to achieve precisely dosed plasma derivatives.
Moreover, advanced filling systems can avoid underfilled vials. If the weighing process reveals that certain containers do not yet hold the required amount of product, the filling needles swing to the corresponding containers to top them up.
Isolators for optimum aseptic conditions
Sensitive plasma derivatives are typically filled in isolators or RABS to ensure their safety. Both approaches to separate operating personnel and products have become even more important considering the revised version of EU GMP Annex 1.
In terms of aseptic production, Annex 1 explicitly recommends both technologies in combination with a higher degree of automation to increase the quality of medicinal products. This includes effective biodecontamination prior to the actual filling process.
Isolator systems from leading manufacturers use vaporised hydrogen peroxide (H2O2) as part of a multistage process that includes preparation, conditioning and aeration in addition to biodecontamination.
The goal is a six-log reduction of the biological indicator organism. The residual concentration should be less than 1 ppm (part per million) as proteins react sensitively to vaporised hydrogen peroxide; residual concentrations lower than 1 ppm can also be achieved.
Fail-safe freeze-drying
The freeze-drying of plasma derivatives is characterised by long processing times. This alternative to the liquid dosage form has become the preferred method to preserve sensitive coagulation factors.
Cycle times of several days are a common requirement for this production step, which makes it even more important to ensure safe, monitored processes. Systems with redundant and high-quality components support fail-safe production.
Modern freeze-dryers also rely on sophisticated state-of-the-art sensor technology to keep key process parameters such as temperature and pressure stable. Advanced systems with optimised secondary processes such as CIP, SIP, filter sterilisation and WIT (water intrusion test) can save energy, time and media.
Inspection for maximum product quality
Freeze-drying is followed by another important step: the visual inspection of liquid or lyophilised products. This checks for the presence of particles, cosmetic defects and container closure integrity testing (CCIT).
State-of-the-art inspection systems efficiently combine the two processes on a single platform. Leak testing for lyophilised products is carried out using headspace analysis (HSA).
Pasteurisation in keeping with strict criteria
Some products require pasteurisation, during which they are processed as gently as possible in tightly sealed containers and within strict temperature limits.
As a rule, plasma derivatives are pasteurized at 60 °C (140 °F), with deviations of only half a degree (approx. 32 Fahrenheit) permitted during a period of up to 12 hours.
In addition to flow-optimised pasteurisers with corresponding baffles, suitable processes are key to maintaining the right temperature. The steam-air mixture principle provides a homogenous flow through the pasteuriser by ensuring consistent conditions during extended processing times and within a narrow temperature range.
The ideal system configuration can be determined by simulating the process and comparing it with real values — an aspect that makes equipment manufacturers’ process expertise particularly important.
These suppliers involve customers in the analysis and design at an early stage. The same applies to the entire process: by working together closely from the outset, pharmaceutical companies and equipment manufacturers can define and implement optimum systems for precise processes throughout the value chain.
This ensures that patients gain the most from every drop of precious plasma derivatives.
