Many of the workflows in the pharma and biopharma industry are carried out under controlled conditions in cleanrooms. But are these facilities always designed in the best possible way?
In particular, the temperature distribution within cleanrooms with critical processes often presents a challenge: too low or too high temperatures can disturb the cell growth, for example. These difficulties can be avoided as early as the design phase thanks to computational fluid dynamics, best known as CFD analysis. This analysis offers many advantages for the engineering of cleanrooms.
The various technologies associated with cleanrooms are in high demand worldwide. In biopharma, cleanrooms are where life-saving pharmaceuticals are made: drugs that fight cancer or autoimmune diseases, for example.
According to a forecast by the research institute Markets and Markets, the market volume for cleanroom technologies is expected to hit roughly US$ 5.7 billion in 2019. By 2024, it is forecast to grow to as much as $7.9bn. The coming years will see increased investment in cleanrooms, according to Markets and Markets.
When it comes to designing new rooms or even modernising existing ones, CFD analysis helps to create the best conditions possible and, in turn, optimises the production of biologic agents in cleanroom environments.
In the wake of the expected boom of cleanroom construction, the CFD analysis can be employed to plan and engineer new cleanrooms from the ground up optimally. Furthermore, it can facilitate the modernisation of already existing cleanrooms.
CFD is a technique from the field of fluid mechanics and uses computer-based simulations to calculate and visualise temperature and velocity distribution or any other characteristics of fluids
Computational fluid dynamics is a technique from the field of fluid mechanics and uses computer-based simulations to calculate and visualise temperature and velocity distribution or any other characteristics of fluids.
Some common applications for CFD analysis are in the process of designing aircraft, automobiles and ships. Here, computer simulation is used to test the prevailing air or water resistance and to use this to improve the design in order to reduce fuel costs. This makes CFD analysis a more flexible, expeditious and cost-effective approach than experiments in a wind or water tunnel. The method is of great value in other areas as well, namely in the conception of cleanrooms.
CFD for biopharma cleanrooms
A typical application scenario for CFD analysis in the biopharma industry is the process of designing a “hot room” with a Cleanroom Class B rating. This is a perfect example of a facility that must conform to strict parameters.
Hot rooms are used to handle and store viruses, cells and other cultures that can only survive within a narrow temperature range. The most critical factor, therefore, is that the temperature in all areas of the cleanroom is kept high and as stable as possible to ideally stimulate the growth of microorganisms. The temperature must be maintained regardless of outside weather conditions.
In the biopharma industry, a constant temperature of 37oC is often regarded as the required benchmark for optimal microorganism growth.
The maximum permissible range of temperature for growth is often called the “danger zone”, and it is highly dependent on the microorganism that is being grown in the room. Any larger deviations from these optimum will reduce the cleanroom’s effectiveness. Thus, the climate-control system of a cleanroom will be expected to ensure a constant temperature distribution. But how does one plan the climate system of a cleanroom and how exactly can CFD analysis help in the process?
How CFD analysis works
CFD analysis significantly improves the design, validation and efficiency of cleanrooms. This is because modelling a cleanroom’s flow characteristics allows simulation of multiple processes that need to be performed simultaneously inside the room.
Flow characteristics include the distribution of temperature resulting from conduction and convection phenomena, and flow patterns resulting from HVAC inlet and outlet conditions, namely velocity, temperature and location.
To accurately model these patterns, the mathematical equations that govern these processes are solved as part of the CFD analysis. The CFD analysis produces useful technical data that can be integrated into the planning or modernisation of cleanrooms, thereby helping to achieve and consistently maintain the target temperature in all areas of the room.
The CFD analysis produces useful technical data that can be integrated into the planning or modernisation of cleanrooms
CFD analysis should not be seen a separate process, but a part of a wide-ranging approach for the conception of cleanrooms, one that is generally implemented by multi-disciplinary teams.
At Bilfinger, for example, specialists in CFD analysis work together with civil engineers, HVAC engineers, mechanical engineers and process engineers to arrive at a holistic solution. As a team, they all pursue the same goal: to create the perfect design for a cleanroom.
A design and build strategy
The development of a cleanroom entails several well-defined steps. In the design phase, the first order of business is to write a URS (User Requirement Specification) that identifies the working parameters that the cleanroom will have to fulfil as well as any additional requirements that the customer may have. On this basis, a functional specification and a detailed design will be elaborated conforming to specific industrial and equipment-related standards. Once such a detailed design has been prepared, construction of the cleanroom can commence.
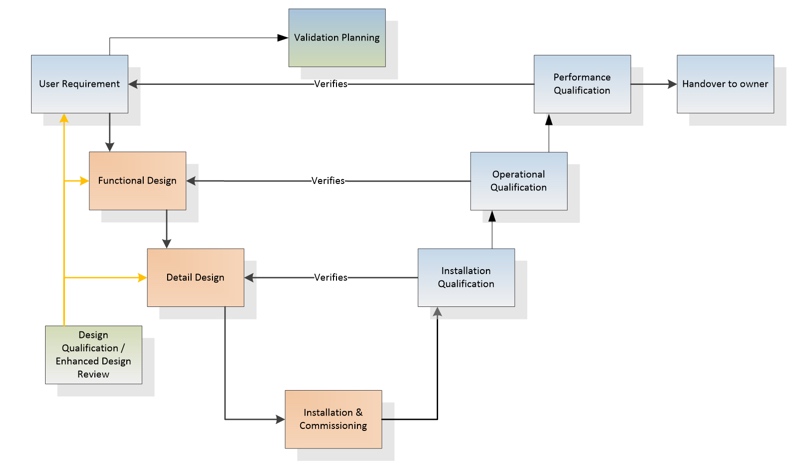
The V Model ensures that a cleanroom is correctly designed for the intended use and also fulfils
all the relevant good practice (GxP) requirements
The testing of the project also unfolds in a systematic, step-by-step manner. In the pharmaceuticals and biopharma industry, all aspects of the design work are typically based on the well-known V Model, which is employed for Design Qualification (DQ) as shown in figure 1. The V Model ensures that a cleanroom is correctly designed for the intended use and also fulfils all the relevant good practice (GxP) requirements, including those of the EU and the US Federal Drug Administration (FDA).
CFD analysis in the V Model
Computer analysis facilitates and improves the workflows of the V Model. It is not always possible to predict during the design phase what conditions will actually look like in the finished cleanroom. This means that there is no absolute certainty whether the cleanroom will ultimately deliver everything that the customer expects.
With CFD analysis, all the relevant details can be simulated in advance during the design phase. To this end, a precise 3D model of the planned cleanroom is created.
The data fed into the model includes the dimensions of the room, the intended positions of the equipment, the location of the climate-control system and other boundary conditions. These calculations allow the flow patterns and flow velocity of the air inside the cleanroom to be simulated. Thus, the model simulates the airflows from the HVAC system, any undesirable turbulence, as well as the areas where the air recirculates and the areas where the flow speed is low.
The simulation, in turn, allows the temperature and velocity distribution to be visualised as well. Figure 2 shows the mean velocity field and flow pattern in the central vertical plane in a cleanroom.
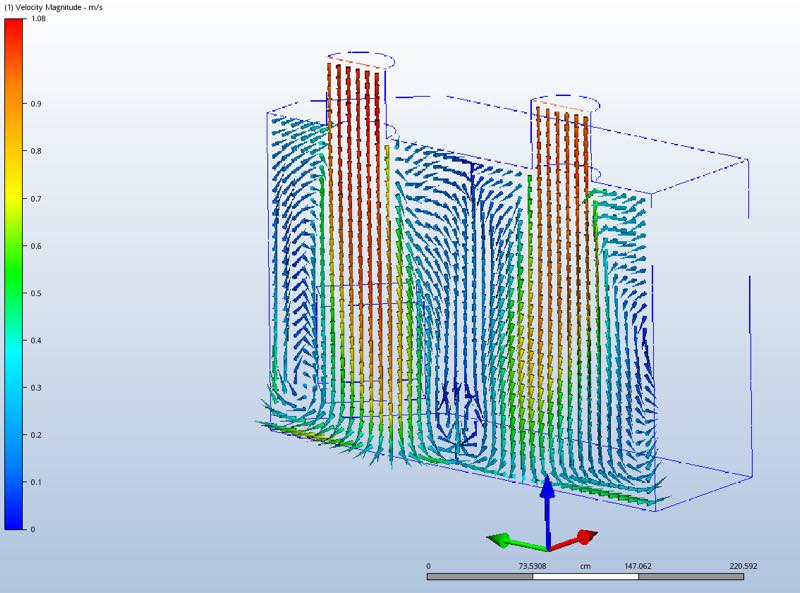
CFD analysis allows the simulation of temperature and velocity distribution to be visualised
Areas where the flow speed is low tend to be characterised by below-target temperatures, which negatively impact the microbiologic growth. Such areas are typically found in proximity to the walls and room corners. But since these areas can be already identified in the planning phase through CFD analysis, there will still be enough time to adapt the cleanroom so as to ensure a better temperature distribution. Moreover, the data generated during the CFD analysis can serve as a guideline for further process validation in the future.
The simulations that form part of CFD analysis require special hardware and software. At Bilfinger, the team uses the Autodesk CFD software. The amount of computer processing power needed could range depending on the simulation's intended scale. This scale will also determine whether the calculations will be performed on local computers or on CPU clusters held available in a cloud environment.
Customer benefits
Given that the design is pre-tested and pre-optimised in a simulation before construction begins, errors or design deficiencies can be eliminated during the design phase and do not have to be corrected at great expense after the cleanroom has already been completed. This approach leads to the smooth execution of the project and also lowers the risk of any potential change during the qualification phase. Plus, the optimal distribution of temperature achieved will also boost the ultimate effectiveness of the cleanroom once it is commissioned.
CFD analysis allows the distribution of temperature across the entire cleanroom to be determined so that the design can be adjusted as required. This makes the technique more precise and detailed than alternative approaches.
The success of a CFD analysis depends on several factors. Its key prerequisites are correct knowledge of the physics of flowing fluids, well-defined boundary conditions, verification of the computational model, and validation of the simulation results to ensure that they are in line with real life.
Generally speaking, CFD analysis is more likely to deliver successful results than experiments designed to test a cleanroom’s prevailing conditions. It is more cost-effective, less time-consuming, provides more information and is safer and more reliable to use.
In contrast to experimental testing, CFD analysis can also be scaled up at will and can be repeated without difficulty. Enterprises in pharma and biopharma that invest in cleanrooms are thus sure to take advantage of CFD analysis in the future with one constant objective in mind: designing the perfect cleanroom.
N.B. This article is featured in the January 2020 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.
