Abstract
One of the major challenges in modern drug development is the poor aqueous solubility of many drug candidates. Nanoparticle formulations are an attractive solution to increase the bioavailability of drugs with low aqueous solubility. Dissolution studies are used to characterize the expected in vivo bioavailability under in vitro conditions. Thus, an efficient dissolution method is necessary during the nanoparticle development process to facilitate the selection of lead formulations with an expected higher bioavailability. The separation of dissolved and undissolved drugs is one of the most important factors in all dissolution studies. In this study, dissolution profiles of ibuprofen nanoparticles are investigated using the Agilent NanoDis system.
Introduction
One of the major challenges in modern drug development is the poor aqueous solubility of many drug candidates. Nanoparticle formulations are an attractive solution to increase the bioavailability of drugs with low aqueous solubility. Dissolution studies are used to characterize the expected in vivo bioavailability under in vitro conditions. Thus, an efficient dissolution method is necessary during the nanoparticle development process to facilitate the selection of lead formulations with an expected higher bioavailability. An efficient method is equally important for quality control purposes during the later stages of the product life cycle. Therefore, the separation of dissolved and undissolved drugs is one of the most important factors in all dissolution studies.
Release medium composition, such as pH and salt concentration, has been shown to influence the dissolution profiles, so the correct selection of the release medium is of major importance. Once the release medium is selected, the solubility of the drug in the release medium needs to be determined to ensure sink conditions for reliable dissolution studies. Sink conditions are achieved once the solubility of the drug in the dissolution medium is at least three times higher than the concentration of the drug in the vessel. Studies not performed under sink conditions may show underestimated release kinetics, which might hide the burst release of the formulation under in vivo conditions.
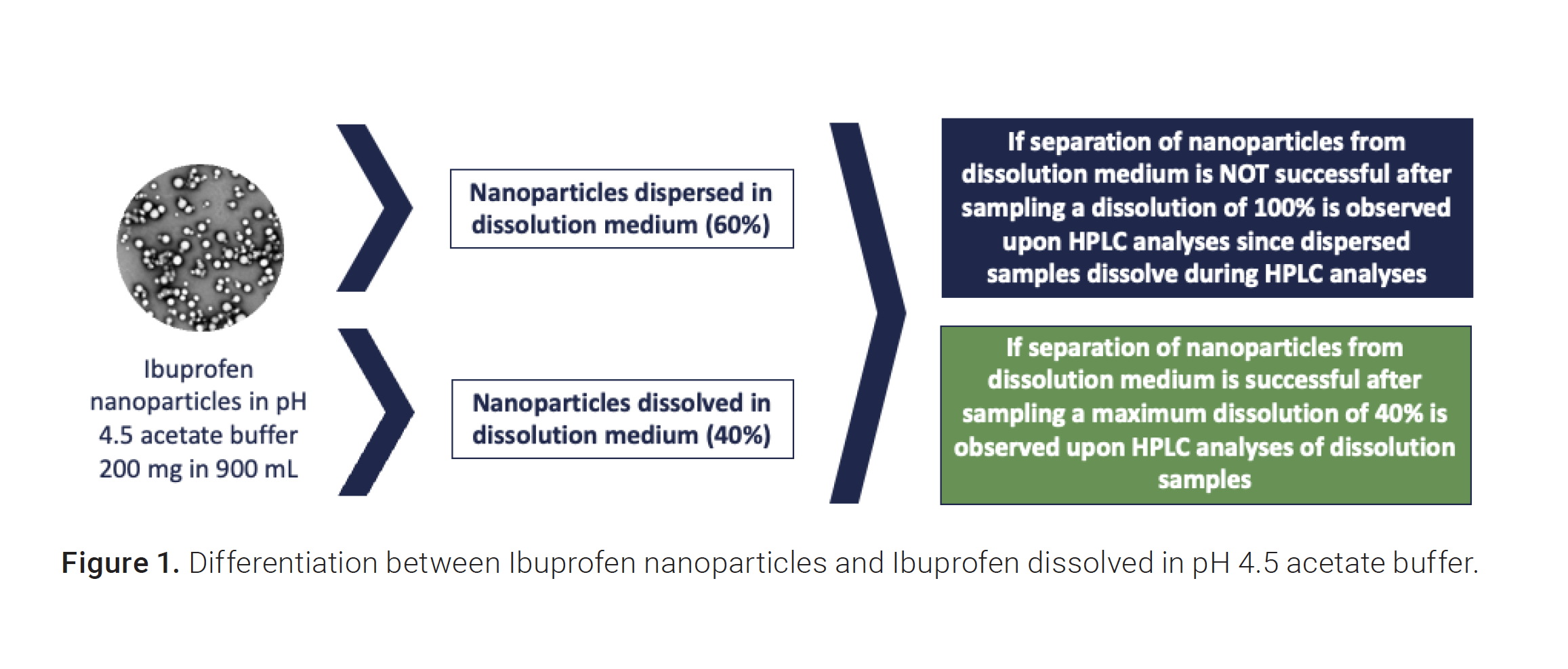
However, nonsink studies may be used if discrimination between the formulations cannot be achieved under sink conditions due to the rapid release kinetics of the loaded drug. Nonsink condition release can therefore prove useful for routine quality control of the formulations and might detect quality changes in the product even before in vivo performance is affected.
In this study, dissolution profiles of ibuprofen nanoparticles are investigated at pH 4.5, where the solubility of ibuprofen is limited. The pH 4.5 buffer was used to test the separation of the nanoparticles from the dissolved API. At pH 4.5, only 40% of the total API concentration in the vessel is dissolved, whereas 60% of the concentration is still in the form of nanoparticles. This reveals the opportunity to understand differences during sample preparation between filtration with standard syringe filters and the crossflow filters in the Agilent NanoDis system. Please see reference 3 for more information on the overall functionality of the Agilent NanoDis platform.
Visit Agilent's Dissolution Testing page
Experimental
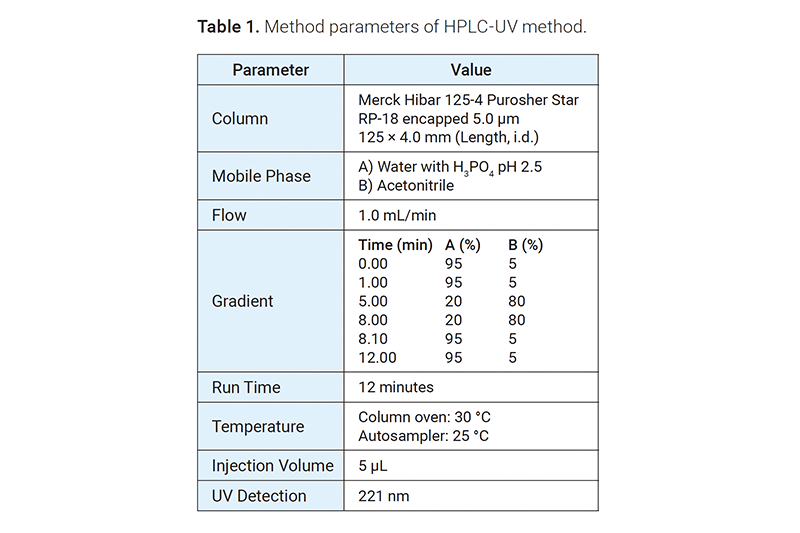
HPLC method for the quantification of dissolution samples
An HPLC-UV method was used for the quantification of the samples.
Particle size measurements were conducted with a Malvern ZS90 Zetasizer. Nanoparticles were diluted 1:20 with distilled water before measuring the particle size.
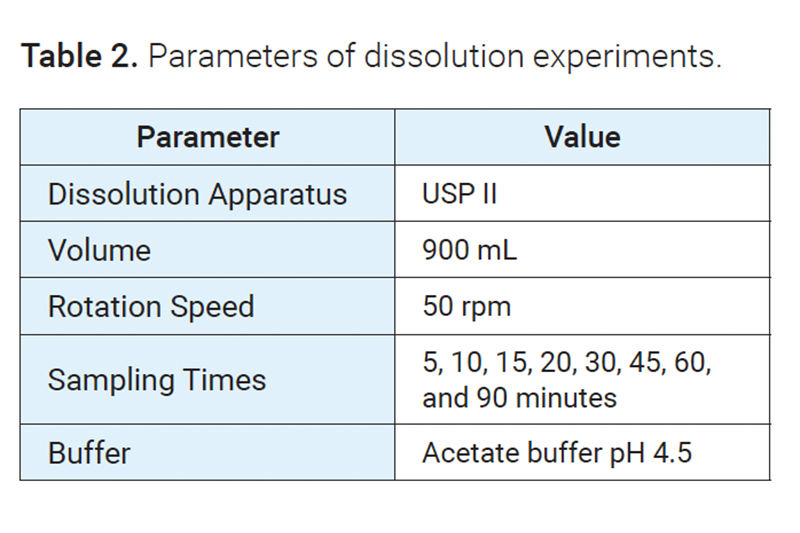
Dissolution method
Dissolution experiments were conducted with an Agilent 708-DS dissolution apparatus coupled with a NanoDis module and an Agilent 850-DS sampling station.
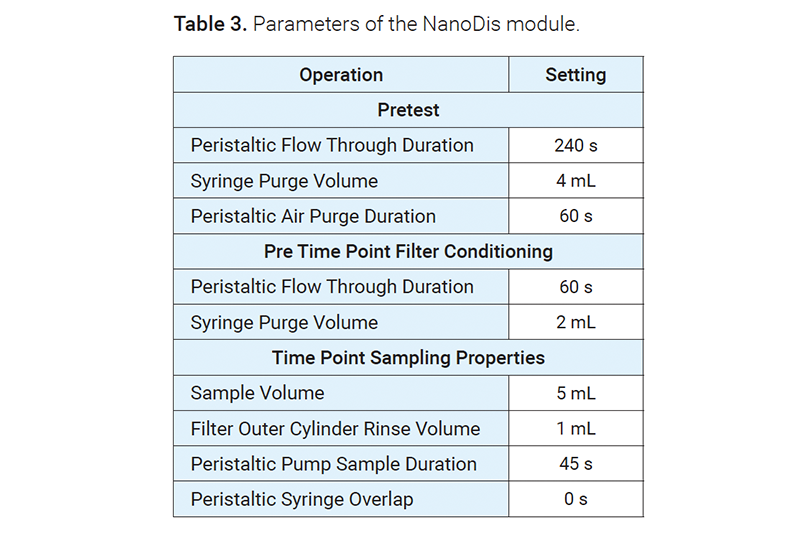
At predetermined time points, the samples were either pulled or filtered by the NanoDis module with 300 kDa mPES membranes. The samples were then collected by the sampling station (850-DS) or filtered manually through 0.02 μm, 0.2 μm, or 0.45 μm PES syringe filters. The whole workflow was controlled by Dissolution Workstation software.
Results and discussion
Particle size characterization of ibuprofen nanoparticles
Three different ibuprofen nanoparticle formulations were prepared, with particle sizes of 112 nm, 187 nm, and 301 nm, and PDI (polydispersity index) values of 0.19, 0.22, and 0.26, respectively.
Dissolution
Dissolution studies were conducted with the 187 nm-sized particles. The performance of syringe filters with different pore sizes and the crossflow filters of the NanoDis were evaluated and compared.
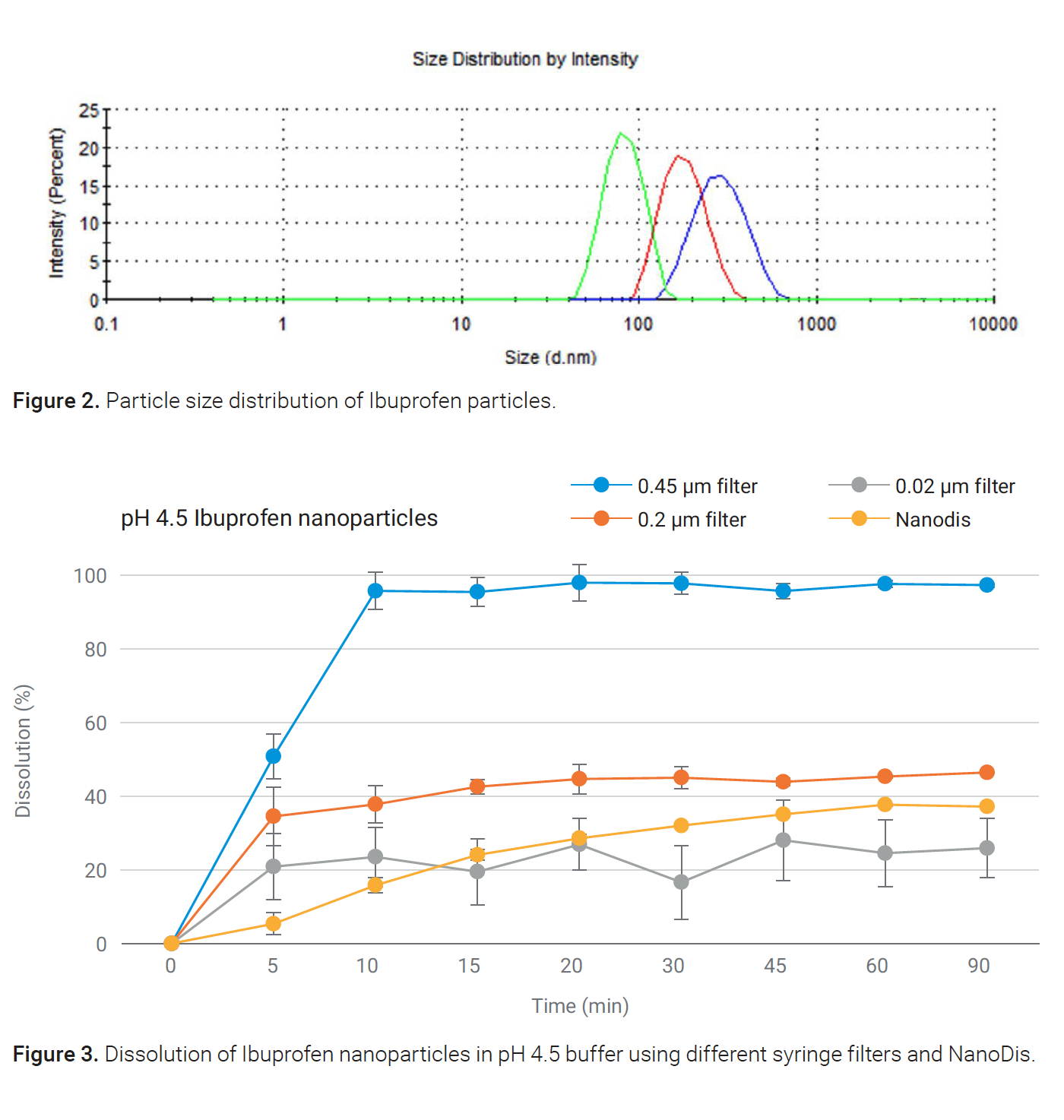
Results showed that 100% dissolution was reached with the 0.45 μm filter, although the API solubility was limited to 40%. This data shows complete permeation of nanoparticles through the 0.45 μm filter. Undissolved particles were then dissolved in the mobile phase during sample analysis, and together with the 40% dissolved API, added up to a 100% recovered API in the dissolution samples. Lower dissolution values were observed for the 0.22 μm filter due to the particle size of ibuprofen, where a significant number of particles are larger than 220 nm. The 0.02 μm filter was blocked during sampling, resulting in a lower and inconsistent dissolution profile. The NanoDis filtration principle enabled the characterization of dissolved API versus undissolved API. Undissolved nanoparticles were completely retained in the filter, allowing for discriminatory dissolution testing.
In the next stage, ibuprofen nanoparticles with particle sizes of 112 nm, 187 nm, and 301 nm were characterized using the NanoDis system and 0.22 μm pore-size syringe filters.
Dissolution profiles gathered with the 0.22 μm syringe filters were dependent on the penetration of the particles through the 0.22 μm filter. The formulation with the smallest particle size of 112 nm showed 100% dissolution within the first time point, since the particles were all smaller than the pore size of the 0.22 μm filter.
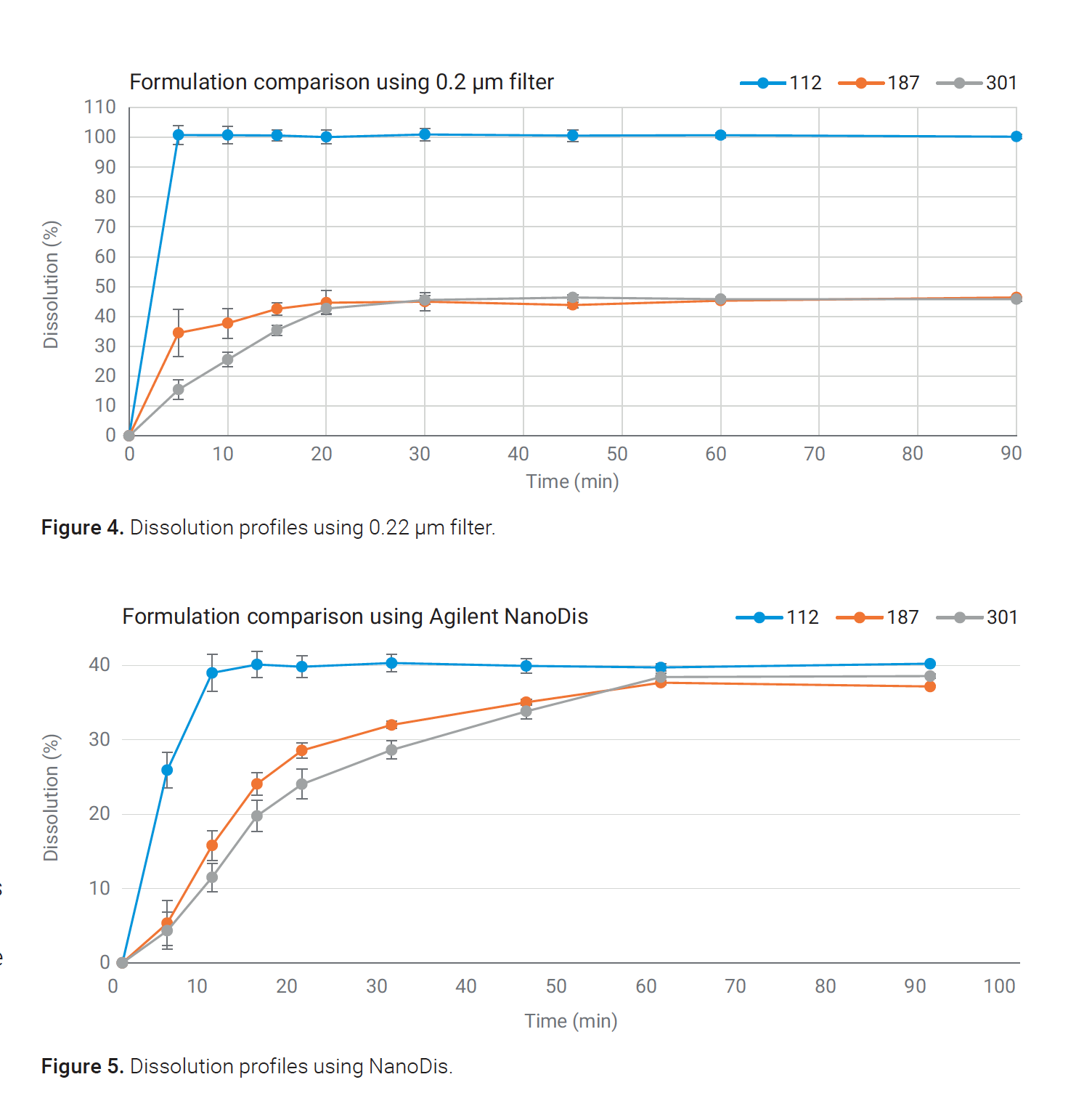
Particles passed through the filter, were dissolved in the mobile phase, and detected by HPLC analysis. The true amount of dissolved API could only be quantified for the 301 nm particle size population since the particle size of most of the particles was higher than 220 nm, and particles were retained by the filter.
Particle size-dependent differences in the dissolution profiles of the ibuprofen nanoparticles were successfully demonstrated using the NanoDis system. The 112 nm particles showed the quickest dissolution profile, whereas there was no significant difference between the 187 and 301 nm particles.
Conclusion
Many techniques have been developed for the release testing of nanoparticles. Recently, more focus has been given to the development of an automated system that could meet ICH guidelines and be used for routine quality control operations. Efficient separation of the free drug from the nanoparticles remains a challenge for most techniques and is essential for the accurate measurement of release. The NanoDis system is able to separate the nanoparticles from the dissolved drug for accurate performance testing of different formulations.
References
- Fattal, E.; Vauthier, C. Drug Delivery: Nanoparticles. Encyclopedia of Pharmaceutical Technology 2007, 2, 1183.
- Faisant, N. et al. Effects of the Type of Release Medium on Drug Release from PLGA-Based Microparticles: Experiment and Theory. International Journal of Pharmaceutics 2006, 314, 189–197, doi:10.1016/j.ijpharm.2005.07.030.
- Agilent NanoDis System Method Development Guide. Agilent Technologies white paper, publication number 5994-2347EN, 2020.
